Table 9.
SARs Associated with Compounds 81 and 9a
| position/scaffold | SAR |
|---|---|
|
| |
|
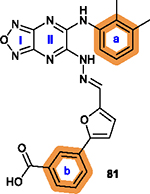
Benzene rings a and b are tolerant of
modification with hydrophobic substituents, such as methyl, nitro, halogen or
carboxylate ester; Other fused ring structures in a are less favorable.20 |
|
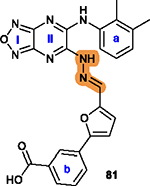
The hydrazone linker is unstable and may undergo
hydrolysis under acidic conditions;21 Its replacement with a secondary amine bond is generally favorable.19 |
|
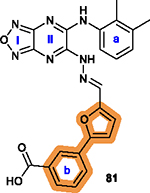
The motif of phenylfuran is tolerable to be replaced.20 |
|
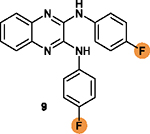
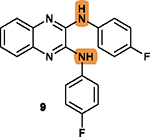
Two fluorine substituents are favorable for
cytotoxicity; Their replacement with electropositive substituent may decrease cytotoxicity;19 |
|
The NHs is necessary to form hydrogen bonds with
residues in the survivin dimeric interface; Its replacement with OH is less favorable.19 |
The highlighted elements are the points of discussion.
