Abstract
Objective Intracranial meningiomas constitute a third of all brain tumors and are among the most common indications for neurosurgical procedures performed worldwide. Most meningiomas present with an indolent, longstanding history. However, the data on outcomes of emergency surgeries for meningioma is limited. This study aims to present our experience of urgent surgical intervention in patients with meningiomas presenting acutely. We also analyze the factors influencing early neurological outcomes and complications.
Materials and Methods All nonelective meningioma surgeries done on an emergency basis between January 2015 and December 2019 were retrospectively reviewed. Patients' demography, clinical, and radiological details were recorded for analysis. The surgical procedure, complications, and follow-up outcomes were also included for statistical comparison.
Results Forty-four patients qualified for the study with a mean age of 49.4 ± 13.4 years. The average presenting Glasgow Coma Scale (GCS) was 13; 47.7% of cases presented with altered sensorium. The most common lesion location was convexity (25, 56.8%), and the mean tumor volume was 74.1 ± 36.5 mL. Gross peritumor edema with mass effect was seen in 16 patients (36.4%). The mean Karnofsky Performance Status at 3 months' follow-up was 89.3 ± 18.2. Patient age and tumor size did not affect outcomes. The presenting GCS of < 15 (odds ratio [OR] 8.8, confidence interval [CI] 0.95–80.72, p 0.03) and the occurrence of postoperative complications (OR 25.71, CI 2.65–249.2, p 0.001) were associated with unfavorable outcomes. Although not statistically significant, a poor tumor grade was also associated with worse clinical outcomes ( p 0.20).
Conclusion Emergency meningioma surgery has comparable outcomes and complication rates with routine elective procedures. Grade II/III meningiomas are more likely to present with acute neurological deterioration and carry a relatively worse prognosis. Poor presenting GCS and postoperative complications are the most critical factors associated with poor patient outcomes in our study.
Keywords: emergency meningioma surgery, emergency neuro-oncology, meningiomas, meningioma surgery outcomes
Introduction
Advancements in the neurosurgical management of meningioma mirror the journey of modern neurosurgery. 1 2 In the words of MacCarty, “ If we were to designate an intracranial neoplasm that has had the most effect on the development of neurologic surgery, very likely the intracranial meningioma would be prominently considered .” 3 Intracranial meningiomas constitute more than one-third of all brain tumors. 4 They are slow-growing tumors, with most having an indolent, longstanding clinical course. Meningioma surgeries are among the most common elective neurosurgical procedures done worldwide. Most tumors are benign, and a gross total resection (GTR) is the surgical goal wherever possible. 5
However, meningiomas can rarely present with acute neurological deterioration (seizures, altered sensorium, or motor deficits). Patients may present with large tumors with mass effect and impending herniation requiring urgent surgical intervention. 6 7 Data on the outcomes and complications of meningioma surgeries done on an emergency basis is limited. This study aimed to present our experience of urgent surgical intervention in patients with meningiomas presenting as an emergency. We also analyze the factors influencing early neurological outcomes and complications.
Materials and Methods
We did a retrospective chart review of all cases with histopathologically proven meningiomas operated on an emergency basis at our hospital between January 2015 and December 2019. These were unplanned and nonelective procedures done on an emergency basis, either because the lesion caused the severe mass effect, perilesional edema, and herniation (temporal/central or subfalcine) with deterioration of sensorium, or there was a large lesion causing impending herniation for which emergency surgery was deemed necessary. The patient's clinical and demographic details were reviewed from the hospital's medical records. All imaging studies of these patients were reviewed. Surgical details were recorded from the database. Postoperative hospital events, discharge, and follow-ups were reviewed.
At presentation, patients presenting to the emergency room (ER) were assessed neurologically and stabilized. Antiedema measures (steroids, mannitol) were started immediately. All patients underwent an urgent computed tomography (CT) brain with contrast, and findings such as lesion location, size, and mass effect were recorded. The attending consultant neurosurgeon decided on the need for urgent surgical decompression. The attending consultant performed all surgeries with the Chief Resident on call in the operating rooms of the emergency and trauma center. Postoperative management was done in the recovery or intensive care unit depending upon the patient's neurological status. A CT brain was repeated 6 to 12 hours after the surgery or immediate postop in the event of any complications. Postop recovery, complications, reexplorations, improvement in sensorium or deficits, and extent of resection were recorded. GTR was defined as complete macroscopic tumor removal. Simpson's grading was used to analyze the extent of resection. 5
Statistical Analysis
We analyzed our cohort's demographic, clinical, surgical details, and perioperative complications. The statistical analysis was conducted using the SPSS software (version 20, SPSS, Inc.). Demographic information and complications were tabulated in numbers and percentages. For univariate analysis, we categorized age (< 65 vs. ≥ 65 years), gender (male vs. female), Glasgow Coma Scale (GCS) (15 vs. < 15), limb weakness (present vs. absent), the volume of the tumor (< 74 vs. ≥ 74 mL; the mean volume of the lesions included was 74 mL), peritumoral brain edema (PTBE) (present vs. absent), the extent of resection (GTR vs. subtotal resection), complications (present vs. absent), and histopathology (World Health Organization [WHO] grade 1 vs. II/III). The Karnofsky Performance Status (KPS) was used to assess the outcome at 3 months. KPS < 70 was considered unfavorable, and KPS > 70 was favorable. Univariate analysis was performed with the above-said variables to assess the individual influence on the outcome. The odds ratio (OR) and its corresponding 95% confidence interval (95% CI) were computed. Regression analysis was done based on the p -value ≤ 0.2 in the univariate analysis. A p -value of < 0.05 was considered statistically significant.
Results
Patient Demography and Clinical Presentation
During the study period, 44 patients underwent emergency craniotomy and meningioma resection. The mean age of the patients was 49.4 ± 13.4 years (range 21–70 years), and the male-to-female ratio was 1:1 (22 each). At the time of presentation, the average GCS was 13, with 47.7% (21/44) of patients presenting with altered sensorium. Motor weakness was present in 45.5% (20/44) as a primary symptom. The mean KPS at the time of presentation was 58.4 ± 20.3.
The most common lesion location in descending order was convexity (25, 56.8%), parasagittal or falcine (12, 27.3%), skull base (06, 13.6%), and intraventricular (01, 02.3%). The mean tumor volume was 74.1 ± 36.5 mL (range 24–180) calculated on a preoperative contrast-enhanced magnetic resonance imaging or CT brain. Gross PTBE causing mass effect was seen in 16 patients (36.4%) ( Table 1 ).
Table 1. Demographic details of the patients included in the study.
| Variable | Value ( n = 44) | % |
|---|---|---|
| Age (mean, y) | 49.4 ± 13.4.1 (range 21–70) | |
| Sex | Male: 22, Female: 22 | |
| Mean GCS at presentation | 13 | |
| Altered sensorium | 21 | 47.7 |
| Motor weakness | 20 | 45.5 |
| Location: Convexity | 25 | 56.8 |
| Parasagittal | 12 | 27.3 |
| Skull base | 06 | 13.6 |
| Intraventricular | 01 | 2.3 |
| Mean tumor volume (mL) | 74.1, range: 24–180 | |
| Edema | 16 | 36.4 |
| EOR: GTR | 38 | 86.4 |
| STR | 06 | 13.6 |
| HPE: WHO Gr: 1 | 21 | 47.7 |
| WHO Gr: 2 | 21 | 47.7 |
| WHO Gr: 3 | 02 | 04.5 |
Abbreviations: EOR, extent of resection; GCS, Glasgow Coma Scale; GTR, gross total resection; HPE, histopathological examination; STR, subtotal resection; WHO, World Health Organization.
Complications and Outcomes
The mean postop hospital stay was 7.8 ± 5.1 days. The mean KPS at 3-month follow-up was 89.3 ± 18.2 (range 60–100). Operative site hematoma was the most common complication in the postoperative period, found in 07 (15.9%) patients. One of them required immediate reexploration and evacuation ( Fig. 1 ). The rest were managed conservatively. Deterioration of motor power was the second most common complication (06, 13.6%). The deficits were transient in 05 patients and resulted in permanent hemiplegia in 01 patient. Four patients (09.1%) had undergone surgical reexploration; one with postoperative hematoma, one patient with opposite side acute subdural hematoma (SDH) evacuation ( Fig. 2 ), one with neurological deterioration due to increased cerebral edema requiring decompressive craniectomy (DC), and one more where the primary procedure was abandoned due to gross cerebral edema and increased bleeding. The bone flap was not replaced in one patient due to increased intraoperative brain bulge following resection ( Fig. 3 ). Two patients (04.5%) died in the postoperative period (one due to malignant cerebral edema and the other due to infarcts). Adverse events in the postop period are summarized in Table 2 .
Fig. 1.
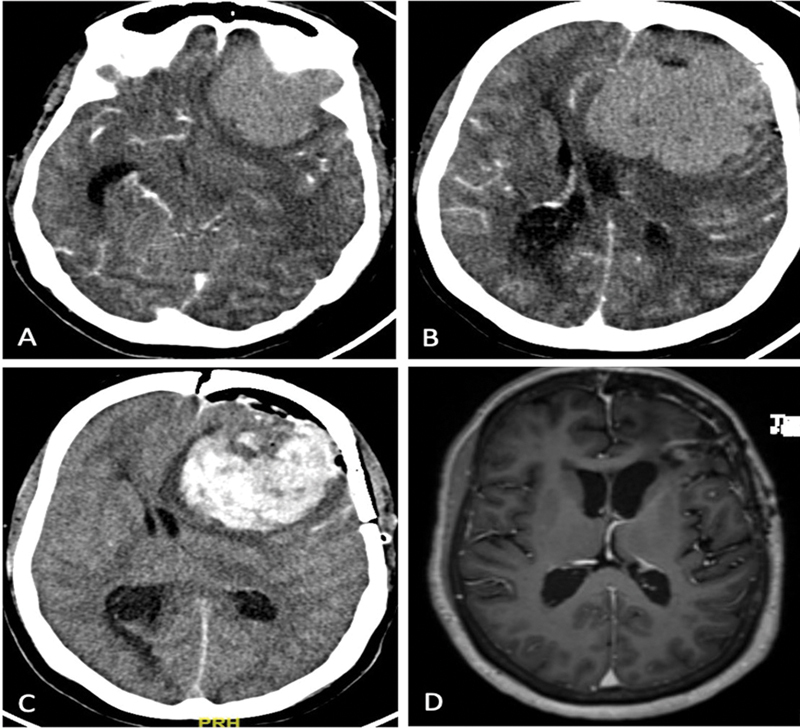
( A , B ) Contrast computed tomography (CT) images of a 54-year-old female patient with left sphenoid wing meningioma operated in the emergency. The patient presented in an altered sensorium with the lesion with edema causing mass effect. ( C ) Postop CT showing operative cavity hematoma. The patient was reexplored with hematoma evacuation. ( D ) Magnetic resonance imaging (MRI) after 3 months showing complete excision of the lesion. The patient had no residual deficits.
Fig. 2.
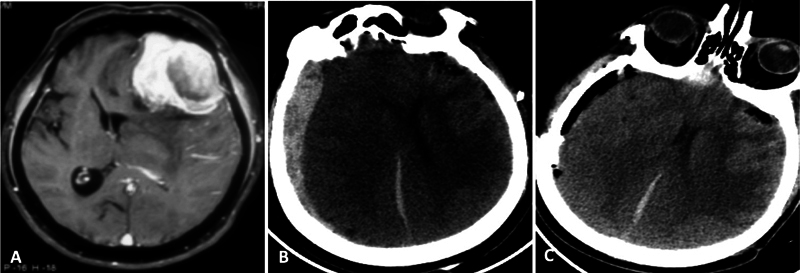
A 68-year-old male patient who underwent emergency craniotomy and resection of left frontal meningioma ( A , contrast magnetic resonance imaging [MRI], axial images). Postop computed tomography (CT) ( B ) showed an opposite side subdural hematoma causing mass effect. ( C ) A right frontotemporoparietal craniotomy was done, and the hematoma was evacuated. The patient made a full functional recovery at 3 months of follow-up.
Fig. 3.
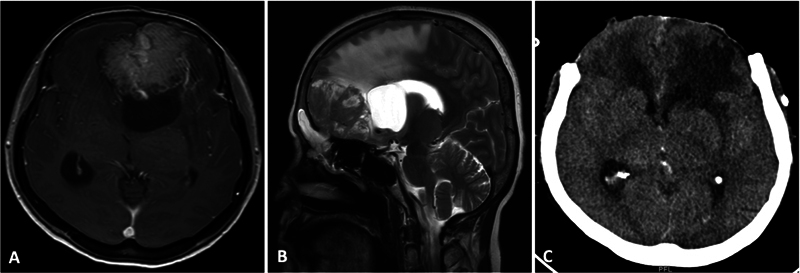
( A , B ) Contrast axial and T2 sagittal magnetic resonance imaging (MRI) of an anterior third falcine meningioma with a loculated posterior cerebrospinal fluid (CSF) cyst, gross peritumoral brain edema (PTBE), and mass effect. ( C ) The patient had a severe intraoperative brain bulge following lesion resection. A decompressive craniectomy was done, and the bone flap was replaced after 3 months.
Table 2. Shows the complications, adverse events, and outcomes following the surgery.
| Complication | Numbers | Percentage |
|---|---|---|
| Postop hematoma - Tumor bed (operative site) Acute SDH |
08 07 01 |
18.2 15.9 02.3 |
| Motor deterioration | 07 | 15.9 |
| Hydrocephalus | 02 | 15 |
| Chest infection | 02 | 15 |
| Progressive cerebral edema | 02 | 15 |
| Seizures | 01 | 02.3 |
| Reexploration | 04 | 09.1 |
| Mean hospital stay (d) | 07.8 ± 05.1 | − |
| Mortality | 02 | 04.5 |
| 3-month, KPS: mean | 86 | Range: 60–100 |
Abbreviations: KPS, Karnofsky Performance Status; SDH, subdural hematoma.
A GTR (Simpson's grade 1) was possible in 38 patients (86.4%). The final histopathological examination showed that 21 patients (47.7%) had WHO grade I and II meningiomas. Two patients died in the hospital, and one patient had an unfavorable outcome with hemiplegia at the time of discharge. The remaining 41 patients had improvement in their presenting functional status (33, 75%) or remained status quo (08, 18.2%). The mean KPS at 3 months' follow-up was 89.3 ± 18.2.
We performed univariate analysis to predict the variables associated with unfavorable outcomes ( Table 3 ). We found that a presenting GCS of < 15 (OR 8.8, CI 0.95–80.72, p 0.03) and the occurrence of postoperative complications (OR 25.71, CI 2.65–249.2, p 0.001) were associated with unfavorable outcomes. Although not statistically significant, a higher tumor grade was also associated with worse clinical outcomes. On regression analysis, only the occurrence of postoperative complications was associated with unfavorable outcomes (OR 18.92, CI 1.75–203.93, p 0.01) ( Table 4 ).
Table 3. Univariate analysis for patients' outcome (KPS scores after the surgery at follow-up).
| Variable | OR | CI | p -Value |
|---|---|---|---|
| Age (< 65 Vs. ≥ 65 y) | 1.03 | 0.10–10.56 | 0.96 |
| Sex (male vs. female) | 0.63 | 0.09–4.21 | 0.67 |
| Presenting GCS (15 vs. < 15) | 8.8 | 0.95–80.72 | 0.03 |
| Limb weakness (present vs. none) | 1.75 | 0.34–8.95 | 0.53 |
| Tumor volume (< 74.1 vs. ≥ 74.1 mL) | 1.38 | 0.26–7.15 | 0.70 |
| PTBE (present vs. none) | 2.77 | 0.53–14.43 | 0.25 |
| EOR (GTR vs. STR) | 3.3 | 0.47–22.97 | 0.28 |
| Complications (present vs. none) | 25.71 | 2.65–249.2 | 0.001 |
| Hematoma (present vs. none) | 0.71 | 0.07–6.92 | 0.84 |
| HPE (WHO Grade I vs. others) | 3.28 | 0.56–19.15 | 0.20 |
Abbreviations: CI, confidence interval; EOR, extent of resection; GCS, Glasgow Coma Scale; GTR, gross total resection; HPE, histopathological examination; KPS, Karnofsky Performance Status; OR, odds ratio; STR, subtotal resection; PTBE, peritumoral brain edema; WHO, World Health Organization.
Table 4. Regression analysis for the outcome with factors having significance ( p < 0.05) as well as histopathology (HPE), p = 0.20 .
| Variable | OR | CI | p -Value |
|---|---|---|---|
| GCS | 8.12 | 0.60–109.43 | 0.14 |
| Complication | 18.92 | 1.75–203.93 | 0.01 |
| HPE | 0.33 | 0.03–3.29 | 0.35 |
Abbreviations: CI, confidence interval; GCS, Glasgow Coma Scale; HPE, histopathological examination; OR, odds ratio.
Discussion
Neglected early symptoms, delay in diagnosis, and awaiting elective surgery are the common reasons for meningioma patients to present to the ER with neurological deterioration. 6 Common causes of neurological deterioration of meningiomas include seizures, increased PTBE causing mass effect, and intratumoral bleeding. 8 9 10 PTBE occurs with more than 50% of all meningiomas. 11 Severe PTBE causing midline shift is a significant risk factor for intraoperative complications and postprocedure morbidity. 10 12 Spontaneous intratumoral or intracranial hemorrhage associated with meningiomas is another reason for acute clinical symptoms and ER visits. 9 13 14 In routine clinical practice, headaches are the most common symptom of brain tumors presenting to the ER. 7 Other features of raised intracranial pressure (ICP) include recurrent vomiting, blurring of vision, altered sensorium, third nerve palsy, or limb weakness due to herniation. Stroke-like presentation, intraventricular bleeding, and hydrocephalus have also been reported with meningiomas. 13
Our study included intracranial meningioma patients who presented with acute neurological deterioration. These cases underwent surgery on an emergency basis, a delay in surgery would cause further deterioration. It included both large tumors with significant mass effect and tumors with severe PTBE causing mass effect. Haq et al mentioned the use of DC at presentation for deep skull base meningiomas with an acute presentation. 6 In another study on DC for intracranial tumors, Jacobo et al found that after emergency DC for meningiomas, the mean postoperative KPS was 70, with a survival rate of 45%. 15 In comparison, the 3-month survival rate was 95.5%, with a mean KPS of 89.3 ± 18.2. We did not prefer a DC in lesions that could be resected in the primary procedure. We also did not encounter any deep, complex skull base lesions requiring emergency procedures during our study period.
A patient's presenting neurological status is a significant predictor of postoperative outcomes. 9 Lesion location (deep midline, posterior fossa) may also affect the occurrence of postoperative deficits after meningioma surgeries. 6 16 Other factors affecting meningioma surgery outcomes include age, with the elderly having more risks of minor complications and poorer functional outcomes. 17 In the current study, the most important factors significantly associated with poor outcomes were poor GCS at the time of presentation and the occurrence of postoperative adverse events ( Table 3 ). Overall, meningioma surgeries carry an increased risk for postoperative hematomas. In a study including 296 meningioma surgeries, Gerlach et al found that an increased risk of hematomas was associated with the elderly population and patients with hematological disorders. They could not find any association between tumor or surgical factors and postoperative hematomas. 18 There were no significant differences in the outcomes of large tumors (> 75 mL volume), tumors with PTBE, and elderly (> 65 years) patients undergoing emergency surgeries ( Table 3 ). Thus, we suggest that advanced age and large tumors should not be contraindications for emergency meningioma surgery, provided the necessary expertise and facilities are available.
In a study on more than 4,000 meningioma surgeries in Japan, Oya et al found that symptomatic patients were more likely to have postoperative complications. They described old age (≥ 65 years), higher grade tumor, tumor at the skull base, size ≥ 30 mm, and subtotal total resections were associated with poor functional outcomes. 19 In another sizeable national meningioma registry from Sweden, including > 2,000 patients, new-onset neurodeficits were seen in 14.8% of patients, reoperations were performed in 5.2% of cases, and 30-day mortality in the whole cohort was 1.5%. 20 Similarly, in a large Norwegian cohort of 1,469 meningioma surgeries, 2.6% of patients had postoperative hematomas, which were significant risk factors for neurologic worsening and 30-day mortality. Lemée et al described early postoperative complications in meningioma surgery to have a negative impact on patient survival (30-day mortality was 5.4%) and postoperative neurologic status. 21 These studies included mostly elective-planned surgeries because of which their conclusions may not be directly correlated with our study. 19 20 21 In contrast, our series had purely emergency meningioma surgeries with postoperative hematoma occurring in eight cases, seven with operative site hematoma and one with opposite acute SDH, probably due to the sudden decompression of ICP causing remote hematoma. 22 Nevertheless, operative site hematomas requiring reexploration (02, 4.5%) and 30-day mortality of 4.5% are comparable to studies with elective surgeries in the extant literature. With the available comparators, it may be concluded that with the available expertise and facilities, meningioma surgeries (even for large tumors) may be safely performed on an emergency basis.
Between 70 and 95% of all meningioma cases undergoing surgery are likely to be WHO grade I. 23 24 However, in the current series, 23/44 (52.27%) patients had a high-grade (WHO grade II or III) lesion, significantly higher than the incidence of these lesions reported in the population. Because of brain invasion, high-grade meningiomas have an increased propensity to present with severe PTBE, 25 26 27 and an increased tendency to intratumoral hemorrhage. 9 These findings suggest that high-grade meningiomas are more likely to present with acute neurological deterioration requiring emergency surgeries in the operating room when compared with grade I meningiomas. There were no patients with intratumoral bleeding in our study. It is to be noted that only cases deemed operable in an emergency operating theater were operated, and the on-call consultant took the decision. Complex skull base tumors (petroclival, cavernous meningiomas, deep posterior fossa meningiomas) which need planned, surgical approaches were not encountered in this study period.
Limitations of the study included retrospective nature, no skull base tumors, and short follow-up available. Meningiomas are commonly operated central nervous system tumors and can present acutely. The results of our study may lack novelty, but they add significantly to the existing literature for emergency surgical management of meningiomas and outcomes.
Conclusion
Surgeries for meningiomas done on an emergency basis have comparable outcomes and complication rates with routine elective surgery. Grade II/III meningiomas are more likely to present with acute neurological deterioration and carry a relatively worse prognosis. Poor presenting GCS and postoperative complications are the most important factors associated with poor patient outcomes in our study.
Conflict of Interest None declared.
Patients' Consent
A written informed consent was taken from the patients for using encrypted patient clinical and imaging details for research and publication purposes.
References
- 1.Patra D P, Savardekar A R, Dossani R H, Narayan V, Mohammed N, Nanda A. Meningioma: the tumor that taught us neurosurgery. World Neurosurg. 2018;118:342–347. doi: 10.1016/j.wneu.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Cushing H. the meningiomas (dural endotheliomas): their source, and favoured seats of origin. Brain. 1922;45(02):282–316. [Google Scholar]
- 3.MacCarty C S, Taylor W F. Intracranial meningiomas: experiences at the Mayo Clinic. Neurol Med Chir (Tokyo) 1979;19(07):569–574. doi: 10.2176/nmc.19.569. [DOI] [PubMed] [Google Scholar]
- 4.Wiemels J, Wrensch M, Claus E B. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(03):307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nanda A, Bir S C, Maiti T K, Konar S K, Missios S, Guthikonda B. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J Neurosurg. 2017;126(01):201–211. doi: 10.3171/2016.1.JNS151842. [DOI] [PubMed] [Google Scholar]
- 6.Haq I BI, Niantiarno F H, Arifianto M R et al. Lifesaving decompressive craniectomy for high intracranial pressure attributed to deep-seated meningioma: emergency management. Asian J Neurosurg. 2021;16(01):119–125. doi: 10.4103/ajns.AJNS_179_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder H, Robinson K, Shah D, Brennan R, Handrigan M. Signs and symptoms of patients with brain tumors presenting to the emergency department. J Emerg Med. 1993;11(03):253–258. doi: 10.1016/0736-4679(93)90042-6. [DOI] [PubMed] [Google Scholar]
- 8.Xue H, Sveinsson O, Tomson T, Mathiesen T. Intracranial meningiomas and seizures: a review of the literature. Acta Neurochir (Wien) 2015;157(09):1541–1548. doi: 10.1007/s00701-015-2495-4. [DOI] [PubMed] [Google Scholar]
- 9.Boŝnjak R, Derham C, Popović M, Ravnik J. Spontaneous intracranial meningioma bleeding: clinicopathological features and outcome. J Neurosurg. 2005;103(03):473–484. doi: 10.3171/jns.2005.103.3.0473. [DOI] [PubMed] [Google Scholar]
- 10.Vignes J R, Sesay M, Rezajooi K, Gimbert E, Liguoro D. Peritumoral edema and prognosis in intracranial meningioma surgery. J Clin Neurosci. 2008;15(07):764–768. doi: 10.1016/j.jocn.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Hou J, Kshettry V R, Selman W R, Bambakidis N C. Peritumoral brain edema in intracranial meningiomas: the emergence of vascular endothelial growth factor-directed therapy. Neurosurg Focus. 2013;35(06):E2. doi: 10.3171/2013.8.FOCUS13301. [DOI] [PubMed] [Google Scholar]
- 12.Missori P, Domenicucci M, Paolini S et al. Emergency decompressive craniectomy after removal of convexity meningiomas. Surg Neurol Int. 2016;7:96. doi: 10.4103/2152-7806.193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumi K, Suma T, Yoshida R et al. Massive intracranial hemorrhage caused by intraventricular meningioma: case report. BMC Neurol. 2021;21(01):25. doi: 10.1186/s12883-021-02056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agazzi S, Burkhardt K, Rilliet B. Acute haemorrhagic presentation of an intracranial meningioma. J Clin Neurosci. 1999;6(03):242–245. doi: 10.1016/s0967-5868(99)90512-x. [DOI] [PubMed] [Google Scholar]
- 15.Jacobo J A, Vazquez-Gregorio R, Moreno-Jiménez S, Mejia-Perez S. Craniectomía descompresiva: un tratamiento de rescate para pacientes con tumores del sistema nervioso central. Cir Cir. 2021;89(05):603–610. doi: 10.24875/CIRU.20000808. [DOI] [PubMed] [Google Scholar]
- 16.Ehresman J S, Garzon-Muvdi T, Rogers D et al. Risk of developing postoperative deficits based on tumor location after surgical resection of an intracranial meningioma. J Neurol Surg B Skull Base. 2019;80(01):59–66. doi: 10.1055/s-0038-1667066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon M TC, Fung L HK, Pu J KS, Leung G KK. Outcome comparison between younger and older patients undergoing intracranial meningioma resections. J Neurooncol. 2013;114(02):219–227. doi: 10.1007/s11060-013-1173-8. [DOI] [PubMed] [Google Scholar]
- 18.Gerlach R, Raabe A, Scharrer I, Meixensberger J, Seifert V. Post-operative hematoma after surgery for intracranial meningiomas: causes, avoidable risk factors and clinical outcome. Neurol Res. 2004;26(01):61–66. doi: 10.1179/016164104773026543. [DOI] [PubMed] [Google Scholar]
- 19.Oya S, Ikawa F, Ichihara N et al. Nation-wide brain tumor registry-based study of intracranial meningioma in Japan: analysis of surgery-related risks. Neurol Med Chir (Tokyo) 2021;61(02):98–106. doi: 10.2176/nmc.oa.2020-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corell A, Thurin E, Skoglund T et al. Neurosurgical treatment and outcome patterns of meningioma in Sweden: a nationwide registry-based study. Acta Neurochir (Wien) 2019;161(02):333–341. doi: 10.1007/s00701-019-03799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemée J M, Corniola M V, Da Broi M, Schaller K, Meling T R. Early postoperative complications in meningioma: predictive factors and impact on outcome. World Neurosurg. 2019;128:e851–e858. doi: 10.1016/j.wneu.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Tyagi G, Bhat D I, Devi B I, Shukla D. Multiple remote sequential supratentorial epidural hematomas-an unusual and rare complication after posterior fossa surgery. World Neurosurg. 2019;128:83–90. doi: 10.1016/j.wneu.2019.04.228. [DOI] [PubMed] [Google Scholar]
- 23.Ostrom Q T, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan J S.CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018 Neuro-oncol 202123(12, suppl 2):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kshettry V R, Ostrom Q T, Kruchko C, Al-Mefty O, Barnett G H, Barnholtz-Sloan J S. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro-oncol. 2015;17(08):1166–1173. doi: 10.1093/neuonc/nov069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong T, Bharatha A, Alsufayan R, Das S, Lin A W. MRI predictors for brain invasion in meningiomas. Neuroradiol J. 2021;34(01):3–7. doi: 10.1177/1971400920953417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brokinkel B, Hess K, Mawrin C. Brain invasion in meningiomas-clinical considerations and impact of neuropathological evaluation: a systematic review. Neuro-oncol. 2017;19(10):1298–1307. doi: 10.1093/neuonc/nox071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behling F, Hempel J M, Schittenhelm J. Brain invasion in meningioma-a prognostic potential worth exploring. Cancers (Basel) 2021;13(13):3259. doi: 10.3390/cancers13133259. [DOI] [PMC free article] [PubMed] [Google Scholar]


