Abstract
Objective
We aimed to investigate antiplatelet drug resistance utilizing light transmission-lumiaggregometry (LT-LA) and the Platelet Function Analyzer-100 (PFA-100) in patients undergoing cardiovascular surgery.
Materials and Methods
The study included 60 patients diagnosed with stable coronary artery disease and peripheral vascular diseases that required surgery. Participants were divided into three groups: patients receiving aspirin (ASA) (n=21), patients receiving clopidogrel (CLO) (n=19), and patients receiving dual therapy (ASA+CLO) (n=20). Aggregation and secretion tests by LT-LA and closure time by the PFA-100 were used to measure antiplatelet drug resistance.
Results
Based on the adenosine diphosphate (ADP)-induced aggregation test, 43% of patients were resistant to ASA, 22% to CLO, and 15% to dual therapy. Diabetes, hypertension, and hyperlipidemia were the most commonly identified comorbid disorders. In patients with comorbid risk factors, the median value of platelet aggregation response to ADP was significantly higher in the ASA group than in the CLO and dual therapy groups (p=0.0001). In patients receiving ASA monotherapy, the maximum amplitude of aggregation response to platelet agonists was ≥70% in 43% of patients for ADP and 28% for collagen by LT-LA. Elevated ADP (≥0.29 nmol) and collagen (≥0.41 nmol)-induced adenosine triphosphate release were found by LT-LA in 66% of patients utilizing an ADP agonist and 80% of patients using a collagen agonist undergoing ASA therapy. Closure times obtained with the PFA-100 were normal in 28% of patients using collagen-ADP cartridges and 62% of patients using collagen-epinephrine (CEPI) cartridges who received ASA. Recurrent thrombosis and bleeding were observed in 12 (20%) patients with cardiovascular disease. Three of these individuals (25%) showed ASA resistance with normal responses to ADP-induced aggregation (≥70%) and secretion (≥0.29 nmol), as well as normal CEPI closure times.
Conclusion
Our findings suggest that antiplatelet drug monitoring by LT-LA and PFA-100 may be useful for high-risk and complicated cardiovascular patients.
Keywords: Antiplatelet therapy, Lumiaggregometry, Platelet Function Analyzer-100, Cardiovascular disorder
Abstract
Amaç
Bu çalışmanın amacı kardiyovasküler cerrahi geçiren hastalarda light transmission-lumiaggregometry (LT-LA) ve Platelet Function Analyzer-100 (PFA-100) kullanarak antitrombosit ilaç direncini araştırmaktır.
Gereç ve Yöntemler
Çalışmaya cerrahi gerektiren stabil koroner arter hastalığı ve periferik vasküler hastalık tanısı konan 60 hasta dahil edilmiştir. Katılımcılar üç gruba ayrılmıştır: Aspirin (ASA) alan hastalar (n=21), klopidogrel (KLO) alan hastalar (n=19) ve ikili tedavi (ASA+KLO) alan hastalar (n=20). Antitrombosit ilaç direncini araştırmak için agregasyon, sekresyon ve kapanma zamanının test edildiği LT-LA ve PFA-100 cihazları kullanılmıştır.
Bulgular
Adenozin difosfat (ADP) ile indüklenmiş agregasyon yanıtına göre ASA direnci 21 hastanın %43’ünde tespit edilirken, bunu 19 hastanın %22’sinde KLO direnci ve 20 hastanın %15’inde ikili ilaç direnci izlemiştir. Diyabet, hipertansiyon ve hiperlipidemi en sık tanımlanan komorbid hastalıklardı. Komorbid risk faktörleri olan hastalarda, ADP’ye verilen trombosit agregasyon yanıtının medyan maksimum amplitüdü ASA grubunda KLO ve ikili tedavi gruplarına göre anlamlı olarak yüksekti (p=0,0001). ASA monoterapisi ile tedavi edilen hastalarda, LT-LA’da trombosit agonistlerine karşı agregasyon yanıtının maksimum amplitüdü, ADP agonisti kullanan hastaların %43’ünde ve kolajen agonisti kullanan hastaların %28’inde ≥%70 üzerinde bulunmuştur. ASA tedavisi sırasında LT-LA’da ADP (≥0,29 nmol) ve kolajen (≥0,41 nmol) ile indüklenen artmış ATP salınımı, ADP agonisti kullanılan hastaların %66’sında ve kolajen agonisti kullanılan hastaların %80’inde saptanmıştır. Sadece ASA alan hastaların PFA-100 cihazında kapanma süreleri, kolajen-ADP kartuşu kullanarak %28’sinde ve kolajen-epinefrin (CEPI) kartuşu kullanarak %62’sinde normaldi. Kardiyovasküler cerrahi geçiren hastaların 12’sinde (%20) tekrarlayan tromboz ve kanama tespit edildi. Bu hastaların üçünde (%25) ADP ile indüklenen agregasyon (≥%70) ve sekresyon (≥0,29 nmol) normal yanıtı yanında CEPI kartuşu ile normal kapanma sürelerini içeren ASA direnci bulundu.
Sonuç
Bulgularımız, LT-LA ve PFA-100 ile antitrombosit ilaç takibinin yüksek riskli ve komplike kardiyovasküler cerrahi hastalarında yararlı olabileceğine işaret etmektedir.
Keywords: Antitrombosit tedavi, Lumiaggregometri, Platelet Function Analyzer-100, Kardiyovasküler hastalıklar
Introduction
Preventing recurrent thrombotic events following cardiovascular surgery is crucial for individuals with coronary artery disease (CAD) or peripheric vascular disease (PVD). Aspirin (ASA) and clopidogrel (CLO) have successfully reduced thromboembolic events following cardiovascular surgery in long-term patient follow-up [1]. However, ASA and CLO resistance remain an important barrier in preventing thromboembolic or ischemic events [2]. Platelet function testing is useful in determining antiplatelet drug responses. Several laboratory tests can be used to assess platelet function [3, 4, 5]. For years, classical light transmission aggregometry (LTA) with arachidonic acid (AA) or adenosine diphosphate (ADP) agonists, as well as the VerifyNow® rapid platelet function assay, have been successfully used to monitor ASA and CLO responses. The Platelet Function Analyzer-100 (PFA-100) and serum or urine thromboxane B2 (TxB2) release levels can only be used to monitor ASA responses. The stability of the P2Y12 receptor to the vasoactive-induced phosphorylation (VASP) test is used to monitor CLO responses. Recently, light transmission-lumiaggregometry (LT-LA) using platelet-rich plasma has been applied to utilize platelet secretion concurrently with platelet aggregation for some inherited or acquired platelet function disorders [6]. However, few studies have reported ASA responses utilizing LT-LA in individuals with CAD [7, 8, 9]. Furthermore, there is only one investigation to date analyzing responses to ASA, CLO, and dual therapy using whole blood impedance aggregometry in patients with acute coronary syndrome who require stents [10]. No study has been found that investigates responses to ASA, CLO, and dual therapy using LT-LA and the PFA-100 in patients with both CAD and PVD after surgery.
This study aimed to investigate antiplatelet drug resistance using LT-LA and the PFA-100 in patients undergoing cardiovascular surgery.
Materials and Methods
This cross-sectional study was carried out in a cardiovascular surgery clinic from January 2023 to December 2023. It was approved by the Ethics Committee of the Gazi University Faculty of Medicine (approval date: 26.12.2023, decision number: 22). Informed consent was obtained from the patients.
Study Groups
The study included 60 consecutive patients with stable CAD and PVD presenting to the cardiovascular surgery clinic. Patients with bleeding disorders, atrial fibrillation, critical limb ischemia, laboratory abnormalities (hemoglobin levels of <10 g/dL, platelet counts of <100,000/µL), or age of <18 years were excluded. Diabetes mellitus (DM), hypertension (HT), hyperlipidemia (HPL), deep venous thrombosis, and cerebrovascular diseases were identified as comorbidities. Anti-DM medications, anti-HT medications, and statins were administered before and after surgery to control DM, HT, and HPL, respectively. Patients with PVD were given cilostazol or non-steroidal anti-inflammatory drugs to alleviate leg pain before surgery. These medicines were discontinued at least 1 week before the operations. None of the patients received pentoxifylline before or after surgery.
Patients were divided into three groups based on their antiplatelet drug use, with ASA monotherapy in group 1 (n=21), CLO monotherapy in group 2 (n=19), and dual therapy (ASA+CLO) in group 3 (n=20).
Study Design
According to our institutional policy, patients received dual therapy with ASA and CLO for at least 6 months for PVD and 12 months for CAD after surgery, followed by ASA or CLO monotherapy in long-term follow-up. If the patient experienced gastrointestinal intolerance or other adverse reactions to ASA, CLO was initially administered. If adverse effects did not occur, ASA was initially administered as monotherapy. CLO was started at the standard dose without a loading dose due to the risk of bleeding 24 h after surgery. All patients with CAD and PVD were given 100 mg of enteric-coated ASA and 75 mg of CLO daily following surgery.
Platelet Function Testing
Platelet function testing was performed for patients who had taken ASA and/or CLO for at least 15 days using the Chrono-Log 700 LA device (Chrono-Log, Havertown, PA, USA) and the PFA-100 (Dade Behring, Deerfield, IL, USA) in the hemostasis laboratory. Blood samples were drawn into 3.8% vacutainer citrate tubes and transferred to the hemostasis laboratory within 1 h of collection. Platelet aggregation and secretion were measured using LT-LA. The maximum amplitude of response to ADP (5 µM) and collagen (2 µM) agonists was recorded. Additionally, adenosine triphosphate (ATP) release was assessed in response to ADP (5 µM) and collagen (2 µM) agonists. The closure times for collagen-ADP (CADP) and collagen-epinephrine (CEPI) cartridges in the PFA-100 device were measured. According to our laboratory reference values, the upper limits for CADP and CEPI are 125 and 157 s, respectively.
Antiplatelet Drug Response
ASA resistance was defined as the maximum amplitude of platelet aggregation response to ADP (≥70%) and the maximum amplitude of platelet aggregation response to collagen (≥70%) by LT-LA in the ASPECT study [8]. Platelet secretion defects were defined as decreased or absent ATP release in response to one or more agonists, with or without defective aggregation tests. The lower reference limit for ATP release produced by ADP was 0.29 nmol and that of collagen was 0.41 nmol in healthy adults receiving ASA [11]. Based on our laboratory reference values, ASA resistance was defined as a closure time of less than 157 s using CEPI cartridges and less than 125 s using CADP cartridges in the PFA-100 device.
For CLO resistance, based on the maximum amplitude of platelet aggregation response to ADP, platelet dysfunction was classified as follows: normal function (70%-100%), mild dysfunction (50%-69%), moderate dysfunction (40%-49%), or severe dysfunction (≤39%) [12].
Dual antiplatelet resistance (ASA and CLO) was defined as ADP-induced platelet aggregation of ≥70% [13].
Statistical Analysis
All data were analyzed using IBM SPSS Statistics 20.0. Variables were examined using the Kolmogorov-Smirnov test to determine whether they were normally distributed. Descriptive analyses were conducted using medians and interquartile ranges for non-normally distributed data. The Mann-Whitney U test was used to compare ADP-induced aggregation between groups. Significance was accepted at p<0.05.
Results
This study included 60 consecutive patients with a median age of 62.5 years, 74% of whom were male while 26% were female. CAD was observed in 46% of patients and PVD in 54%. The common comorbid conditions included DM (42%), HT (36%), HPL (16%), deep venous thrombosis (8%), and cerebrovascular disease (5%). Patients were prescribed ASA monotherapy (35%), CLO monotherapy (32%), or dual therapy (33%) after surgery. Complications (recurrent thrombosis/bleeding) and death were observed in 20% and 4% of the total cases, respectively (Table 1).
Table 1. Demographic data.
|
Variables (n=60) |
|
|
Age, years, median (range) |
62.5 (43-84) |
|
Sex, male/female |
44 (74%)/16 (26%) |
|
Antiplatelet indication |
|
|
Coronary artery disease - yes/no |
28 (46%)/32 (54%) |
|
Peripheric vascular disease - yes/no |
32 (54%)/28 (46%) |
|
Comorbid conditions |
|
|
Diabetes mellitus - yes/no |
25 (42%)/35 (58%) |
|
Hypertension - yes/no |
22 (36%)/38 (64%) |
|
Hyperlipidemia - yes/no |
10 (16%)/50 (84%) |
|
Deep venous thrombosis - yes/no |
5 (8%)/55 (92%) |
|
Cerebrovascular disease - yes/no |
3 (5%)/57 (95%) |
|
Antiplatelet medication |
|
|
Aspirin monotherapy |
21 (35%) |
|
Clopidogrel monotherapy |
19 (32%) |
|
Aspirin and clopidogrel dual therapy |
20 (33%) |
|
Platelet function testing |
|
|
Light transmission lumiaggregometry |
|
|
Aspirin resistance, n=21 |
|
|
Aggregation response in LT-LA |
|
|
ADP-agonist 70%/≥70% |
12 (57%)/9 (43%) |
|
Collagen-agonist 70%/≥70% |
15 (72%)/6 (28%) |
|
ATP secretion in LT-LA |
|
|
ADP-agonist 0.29/≥0.29 |
7 (34%)/14 (66%) |
|
Collagen-agonist 0.41/≥0.41 |
4 (20%)/17 (80%) |
|
PFA-100 |
|
|
Collagen-ADP cartridge 125 s/≥125 s |
6 (28%)/15 (72%) |
|
Collagen-epinephrine cartridge 157 s/≥157 s |
13 (62%)/8 (38%) |
|
Clopidogrel resistance, n=19 |
|
|
Aggregation response using ADP-agonist in LT-LA |
Platelet dysfunction |
|
0%-39% - severe |
9 (48%) |
|
40%-49% - moderate |
5 (26%) |
|
50%-69% - mild |
1 (4%) |
|
≥70% - normal |
4 (22%) |
|
Dual therapy resistance, n=20 |
|
|
Aggregation response in LT-LA |
|
|
ADP-agonist 70%/≥70% |
17 (85%)/3 (15%) |
|
Complications |
|
|
Thrombotic and/or bleeding events - yes/no |
12 (20%)/48 (80%) |
|
Death - yes/no |
2 (4%)/58 (96%) |
LT-LA: Light transmission lumiaggregometry; ADP: adenosine diphosphate; ATP: adenosine triphosphate; PFA-100: Platelet Function Analyzer-100.
Assessment of Antiplatelet Drug Resistance
Based on their ADP-induced aggregation responses, 43% of patients were resistant to ASA, 22% to CLO, and 15% to dual therapy. In patients receiving ASA monotherapy, the maximum amplitude of aggregation response to platelet agonists was ≥70% in 43% of patients for ADP and 28% for collagen. The ADP-induced ATP release was above 0.29 nmol in 66% of patients receiving ASA treatment. Collagen-induced ATP release was above 0.41 nmol in 80% of patients receiving ASA treatment. Closure times were normal in 28% of CADP patients and 62% of CEPI patients on ASA monotherapy. Based on the maximum amplitude of platelet aggregation responses to ADP for CLO resistance, 48%, 26%, 4%, and 22% of patients receiving CLO monotherapy had severe, moderate, or mild platelet dysfunction and normal function, respectively. In patients undergoing dual therapy, the maximum amplitude of aggregation response to the ADP platelet agonist was ≥70% in 15% of patients (Table 1). Figures 1 and 2 show the numbers of patients for whom antiplatelet drug responses were measured by LT-LA and the PFA-100. In patients with comorbid conditions, the median maximum amplitude of platelet aggregation response to ADP was significantly higher in the ASA group at 74% (range: 50%-90%) than in the CLO group at 39% (range: 20%-55%) or the dual therapy group at 47% (range: 33%-57%) (p=0.0001). There were no significant differences between the CLO and dual treatment groups (p=0.21) (Figure 3).
Figure 1.
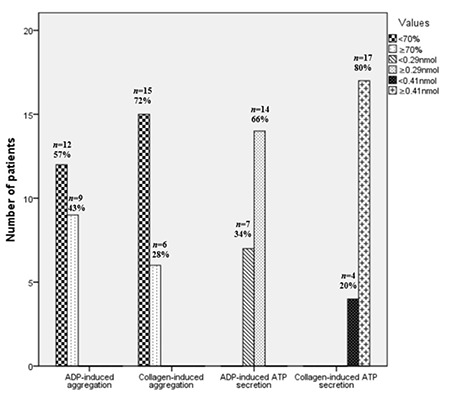
Number of patients receiving aspirin in assessments of platelet aggregation and secretion using ADP and collagen agonists in LT-LA.
LT-LA: Light transmission lumiaggregometry; ADP: adenosine diphosphate; ATP: adenosine triphosphate.
Figure 2.
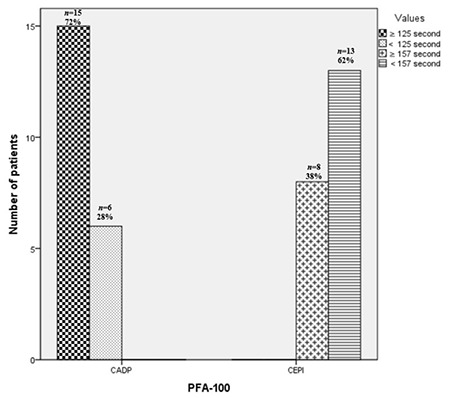
Number of patients receiving aspirin in assessments of closure times using collagen-ADP (CADP) and collagen-epinephrine (CEPI) cartridges with the PFA-100.
ADP: Adenosine diphosphate; PFA-100: Platelet Function Analyzer-100.
Figure 3.
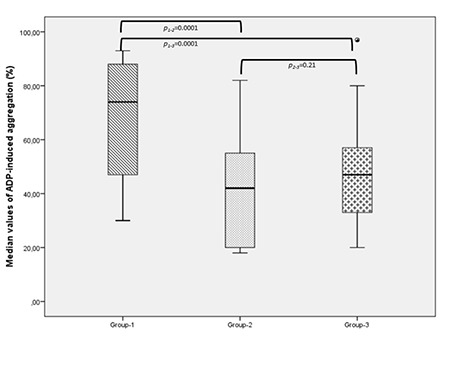
Comparison of antiplatelet drug response based on ADP-induced platelet aggregation using LT-LA in patients with comorbid conditions.
ADP: Adenosine diphosphate; LT-LA: light transmission lumiaggregometry.
Clinical and Laboratory Findings of Complicated Patients Receiving Antiplatelet Drugs
Complications were observed in 12 (20%) patients undergoing cardiovascular surgery. Eight of them had CAD and the remaining four had PVD. Three patients (25%) demonstrated ASA resistance based on the normal response to the amplitude of ADP-induced aggregation using LT-LA and normal closure time utilizing CEPI responses and the PFA-100. Comorbid conditions were observed in all three of those patients, one of whom had CAD while the other two had PVD. These three patients developed recurrent thrombosis. The antiplatelet medication was changed to CLO. They were given anti-HT and anti-DM medications and statins all at the same time. Two patients (16%) died due to bleeding after receiving dual antiplatelet therapy. There was no evidence of resistance to antiplatelet drugs in the two individuals who died of bleeding (Table 2).
Table 2. Clinical and laboratory findings of complicated patients receiving antiplatelet drugs.
|
Age, years/sex |
Antiplatelet indication |
ASA/CLO/dual therapy |
Aggregation ADP ≥70%/collagen ≥70% |
ATP release ADP ≥0.29/collagen ≥0.41 |
PFA-100 CADP ≥125 s/CEPI ≥157 s |
Comorbid conditions |
Complications: thrombosis recurrence/bleedings |
Concomitant drugs |
Changes in antiplatelet drugs |
Death |
|
|
1 |
78/male |
CAD |
-/-/+ |
-/- |
+/+ |
+/+ |
HT/DM |
+/+ |
Anti-HT, antidiabetic |
No |
No |
|
2 |
52/male |
CAD |
-/-/+ |
-/- |
-/+ |
-/+ |
HT/DM |
-/+ |
Anti-HT, antidiabetic |
No |
No |
|
3 |
77/male |
PVD |
-/-/+ |
-/- |
-/- |
-/- |
- |
-/- |
NSAID* |
No |
No |
|
4 |
69/male |
CAD |
+/-/- |
+/- |
+/- |
-/- |
HT/DM/HPL |
+/- |
Statin, anti-HT, antidiabetic |
Yes |
No |
|
5 |
73/male |
CAD |
-/+/- |
-/- |
+/+ |
+/+ |
HT/DM |
+/+ |
Anti-HT, antidiabetic |
No |
No |
|
6 |
72/male |
PVD |
-/-/+ |
-/- |
-/+ |
-/+ |
DM |
-/+ |
NSAID*, antidiabetic |
No |
Yes |
|
7 |
60/male |
CAD |
-/-/+ |
-/- |
+/+ |
+/+ |
DM/HPL |
+/- |
Statin, antidiabetic |
No |
No |
|
8 |
63/male |
CAD |
-/+/- |
-/- |
-/+ |
+/+ |
DM/HPL |
+/- |
Statin, antidiabetic |
No |
No |
|
9 |
66/male |
PVD |
+/-/- |
+/- |
+/+ |
+/- |
DM/HPL |
+/- |
Statin, NSAID*, antidiabetic |
Yes |
No |
|
10 |
64/female |
PVD |
+/-/- |
+/- |
+/+ |
+/- |
HT/DM |
+/- |
Anti-HT, NSAID*, antidiabetic |
Yes |
No |
|
11 |
58/male |
CAD |
-/+/- |
-/- |
-/+ |
+/- |
HT |
-/+ |
Anti-HT |
No |
No |
|
12 |
62/male |
CAD |
-/-/+ |
-/- |
-/+ |
-/+ |
HT/HPL |
-/+ |
Statin, anti-HT |
No |
Yes |
*: Used only before surgery; ASA: aspirin; CLO: clopidogrel; ADP: adenosine diphosphate; ATP: adenosine triphosphate; PFA-100: Platelet Function Analyzer-100; CAD: coronary artery disease; CADP: collagen-ADP; PVD: peripheral vascular disease; HT: hypertension; DM: diabetes mellitus; HPL: hyperlipidemia; CEPI: collagen-epinephrin; anti-HT: antihypertensive; NSAID: non-steroid anti-inflammatory drug.
Discussion
In this cross-sectional study, we performed platelet function testing to evaluate antiplatelet drug responses in patients undergoing cardiovascular surgery. Our main findings were as follows: First, patients receiving ASA had a higher rate of drug resistance than those on CLO or dual therapy as determined using LT-LA and the PFA-100 in patients undergoing cardiovascular surgery. Second, comorbid disease can contribute to the development of ASA resistance in these patients. Third, dual antiplatelet therapy may pose bleeding risks in patients who have no resistance to antiplatelet drugs.
ASA and CLO are the two best known antiplatelet drugs used by atherothrombotic patients [1]. ASA inhibits cyclooxygenase (COX1)-mediated thromboxane production, which is essential for platelet aggregation and activation, while CLO inhibits platelet aggregation and activation by binding the ADP agonist to the P2Y12 receptor on the platelet membrane [14]. Platelet function tests can be used to measure both antiplatelet drug responses. The VASP test is the gold standard for measuring CLO response; however, it is expensive and requires flow cytometry [2]. The COX1-specific standard tests for ASA response are AA-induced LTA and serum TxB2 levels. AA-induced LTA has good specificity but low sensitivity and serum TxB2 is not platelet-specific [2, 15]. In recent years, there has been an increase in research into non-specific COX1 tests, such as ADP-induced aggregation using LT-LA testing [7, 8, 9]. In this study, we used the LT-LA test, which assesses simultaneous platelet aggregation and secretion using various agonists, and the PFA-100 assay to rapidly determine ASA response. In addition, the ADP-induced aggregation test was used to measure ASA, CLO, or both via LT-LA.
In an extensive review encompassing numerous instruments, antiplatelet drug resistance or non-response was reported for 1-45% of patients using both medications, 4-60% of patients using ASA, and 4%-30% of patients using CLO [2]. The highest rate of ASA resistance was found with the PFA-100, whereas the lowest rate was found with AA-induced LTA and serum TxB2 testing in that review. In our study, which used ADP-induced platelet aggregation with LT-LA, ASA resistance was found in 43% of cases, CLO resistance in 22%, and resistance to both drugs in 15%. Similarly, we observed a higher rate (62%) of ASA resistance using the CEPI response with the PFA-100. In a detailed study, the authors demonstrated that prolonged CEPI was a crucial indicator of ASA response [16]. However, the rate of ASA resistance using collagen-induced platelet aggregation (28%) was lower than that of ADP-induced platelet aggregation (43%) in our study. Ohmori et al. [17] showed that collagen-induced aggregation was more sensitive than ADP in assessing ASA responses in a group of Japanese patients using ASA due to cardiovascular events, and it was revealed that collagen as an agonist was more useful in monitoring ASA resistance. The difference in ASA resistance rates is likely to be the result of measurement method variability or a lack of COX1-specific assays. Furthermore, platelet secretion tests are not commonly used to evaluate antiplatelet drug response. In a small number of healthy individuals receiving ASA, a lower reference limit was determined as a result of ADP- and collagen-induced ATP release [11]. In that study, platelet secretion deficiency was defined at values below 0.29 nmol for ADP and 0.41 nmol for collagen-induced ATP release. Parallel to that study, we used the same cut-off values to assess platelet secretion defects for ASA response. Only 34% of the ASA-treated individuals had ADP-induced ATP secretion deficiencies while 20% had collagen-induced ATP secretion defects, indicating that ASA was not sufficient to impair secretion. Similarly, another study found that only 20% of low-dose ASA patients exhibited ADP- and collagen-induced ATP secretion defects [18]. This study concluded that ASA’s suppression of cyclooxygenase was insufficient to prevent collagen-induced ATP release. Our findings indicate that much more research on drug-induced platelet secretion defects is required to confirm their usefulness for assessing antiplatelet drug response. In addition, both ADP- and collagen-induced platelet aggregation can be used to screen for further ASA resistance.
Although the definitive cause of ASA and CLO resistance is unknown, drug incompatibility, drug-drug interactions, genetics, and concomitant diseases may all contribute to the development of drug resistance [4, 19, 20]. A few studies have identified DM, HT, and HPL as the most common risk factors for comorbidities [21, 22]. Similarly, DM and HT were the most common risk factors in our study and the ASA group had a higher median ADP-induced aggregation value, indicating ASA resistance in the presence of comorbid conditions. In a comprehensive study, thromboembolic complications were identified in 24% of patients with ASA resistance [23]. Similarly, we found that 25% of complicated patients (n=3) who experienced recurrent thrombotic events were unresponsive to ASA as measured by platelet aggregation, secretion, and CEPI responses. They were changed to antiplatelet medications. Due to comorbid conditions, they had received concurrent anti-HT/anti-DM/statin treatment. Furthermore, a large population-based cohort study found that the risk of bleeding ranged from 5% to 18% in CAD patients receiving dual or triple antiplatelet drugs [24]. In our study, two (16%) of 12 complicated patients died as a result of bleeding while receiving dual antiplatelet drugs for CAD and PVD. Platelet function tests indicated no evidence of antiplatelet drug resistance in these two cases. Our findings suggest that comorbid risk factors such as DM, HT, and HPL may have contributed to the development of antiplatelet drug resistance and physicians should be aware of the risk of bleeding associated with dual antiplatelet therapy in the presence of comorbid conditions.
Study Limitations
The main limitations of our study were the small sample size, the high cost of genetic testing, and the lack of repeated measurements of aggregation, secretion tests, and closure times using LT-LA and the PFA-100 due to a limited budget. Furthermore, we did not perform COX1-specific tests such as serum TxB2 measurements, AA-induced platelet aggregation, or VerifyNow platelet testing because they were not available in our laboratory. Our study did not evaluate patient treatment compliance or postoperative blood requirements. Future research is needed to assess both COX1-specific and non-specific diagnostic methods as well as patient adherence in large patient populations.
Conclusion
Our findings suggest that in high-risk patients undergoing complicated cardiovascular surgery, initial dual therapy is more beneficial, but in subsequent monotherapy, CLO may be preferred to ASA due to the high resistance rate of the latter. If ASA is the suggested initial antiaggregant, ASA resistance monitoring using LT-LA and the PFA-100 is required.
Ethics
Ethics Committee Approval: The study was approved by the Ethics Committee of the Gazi University Faculty of Medicine (approval date: 26.12.2023, decision number: 22).
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Footnotes
Authorship Contributions
Surgical and Medical Practices: A.Ö., S.T., H.D., B.K., E.N.Y., G.L.O., Z.K.; Concept: A.Ö., H.D., S.T., B.K., E.N.Y., G.L.O., Z.K.; Design: A.Ö., H.D., S.T., B.K., E.N.Y., G.L.O., Z.K.; Data Collection or Processing: A.Ö., S.T., H.D., B.K., E.N.Y., G.L.O., Z.K.; Analysis or Interpretation: A.Ö., S.T., H.D., B.K., E.N.Y., G.L.O., Z.K.; Literature Search: A.Ö., S.T., H.D., B.K., E.N.Y., G.L.O., Z.K.; Writing: A.Ö., G.L.O., Z.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Arockiam S, Staniforth B, Kepreotis S, Maznyczka A, Bulluck H. A contemporary review of antiplatelet therapies in current clinical practice. Int J Mol Sci . 2023;24(13):11132. doi: 10.3390/ijms241311132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Dor I, Kleiman NS, Lev E. Assessment, mechanisms, and clinical implication of variability in platelet response to aspirin and clopidogrel therapy. Am J Cardiol . 2009;104(2):227–233. doi: 10.1016/j.amjcard.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Michelson AD, Bhatt DL. How I use laboratory monitoring of antiplatelet therapy. Blood . 2017;130(6):713–721. doi: 10.1182/blood-2017-03-742338. [DOI] [PubMed] [Google Scholar]
- 4.Wang TH, Bhatt DL, Topol EJ. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur Heart J . 2006;27(6):647–654. doi: 10.1093/eurheartj/ehi684. [DOI] [PubMed] [Google Scholar]
- 5.Tourmousoglou CE, Rokkas CK. Clopidogrel and aspirin in cardiovascular medicine: responders or not--current best available evidence. Cardiovasc Hematol Agents Med Chem . 2008;6(4):312–322. doi: 10.2174/187152508785909483. [DOI] [PubMed] [Google Scholar]
- 6.Pai M, Wang G, Moffat KA, Liu Y, Seecharan J, Webert K, Heddle N, Hayward C. Diagnostic usefulness of a lumi-aggregometer adenosine triphosphate release assay for the assessment of platelet function disorders. Am J Clin Pathol . 2011;136(3):350–358. doi: 10.1309/AJCP9IPR1TFLUAGM. [DOI] [PubMed] [Google Scholar]
- 7.Homoródi N, Kovács EG, Leé S, Katona É, Shemirani AH, Haramura G, Balogh L, Bereczky Z, Szőke G, Péterfy H, Kiss RG, Édes I, Muszbek L. The lack of aspirin resistance in patients with coronary artery disease. J Transl Med . 2016;14:74. doi: 10.1186/s12967-016-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurbel PA, Bliden KP, DiChiara J, Newcomer J, Weng W, Neerchal NK, Gesheff T, Chaganti SK, Etherington A, Tantry US. Evaluation of dose-related effects of aspirin on platelet function: results from the ASA-Induced Platelet Effect (ASPECT) study. Circulation . 2007;115:3156–3164. doi: 10.1161/CIRCULATIONAHA.106.675587. [DOI] [PubMed] [Google Scholar]
- 9.Abdullah WZ, Abu Bakar S, Wan Mohd Zain WS, Yusof Z, Mustaffa R, Hassan R. Aspirin effects on platelets using whole blood tested by platelet aggregometry: a comparative study for test validation in a clinical hemostasis laboratory. Lab Medicine . 2013;44(1):90–96. doi: 10.1309/lm13ur8vgqtncbvq. [DOI] [Google Scholar]
- 10.Li L, Li HY, Qiao R, Yu HY, Zeng H, Gao W, Zhang J. Predictive value of antiplatelet resistance on early stent thrombosis in patients with acute coronary syndrome. Chin Med J (Engl) . 2013;126:626–633. [PubMed] [Google Scholar]
- 11.Trampuš-Bakija A, Jazbec J, Faganel-Kotnik B. Platelet lumiaggregation testing: reference intervals and the effect of acetylsalicylic acid in healthy adults. J Med Biochem . 2020;39(4):422–427. doi: 10.5937/jomb0-24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Bracey AW, Radovancevic R, Cooper JR Jr, Collard CD, Vaughn WK, Nussmeier NA. Clopidogrel and bleeding in patients undergoing elective coronary artery bypass grafting. J Thorac Cardiovasc Surg . 2004;128(3):425–431. doi: 10.1016/j.jtcvs.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Cuisset T, Frere C, Quilici J, Barbou F, Morange PE, Hovasse T, Bonnet JL, Alessi MC. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost . 2006;4(3):542–549. doi: 10.1111/j.1538-7836.2005.01751.x. [DOI] [PubMed] [Google Scholar]
- 14.Maree AO, Fitzgerald DJ. Variable platelet response to aspirin and clopidogrel in atherothrombotic disease. Circulation . 2007;115(16):2196–2207. doi: 10.1161/CIRCULATIONAHA.106.675991. [DOI] [PubMed] [Google Scholar]
- 15.Renda G, Zurro M, Malatesta G, Ruggieri B, De Caterina R. Inconsistency of different methods for assessing ex vivo platelet function: relevance for the detection of aspirin resistance. Haematologica . 2010;95(12):2095–2101. doi: 10.3324/haematol.2010.027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller T, Dieplinger B, Poelz W, Haltmayer M. Utility of the PFA-100 instrument and the novel multiplate analyzer for the assessment of aspirin and clopidogrel effects on platelet function in patients with cardiovascular disease. Clin Appl Thromb Hemost . 2009;15(6):652–659. doi: 10.1177/1076029608322547. [DOI] [PubMed] [Google Scholar]
- 17.Ohmori T, Yatomi Y, Nonaka T, Kobayashi Y, Madoiwa S, Mimuro J, Ozaki Y, Sakata Y. Aspirin resistance detected with aggregometry cannot be explained by cyclooxygenase activity: involvement of other signaling pathways(s) in cardiovascular events of aspirin-treated patients. J Thromb Haemost . 2006;4:1271–1278. doi: 10.1111/j.1538-7836.2006.01958.x. [DOI] [PubMed] [Google Scholar]
- 18.Braun M, Kramann J, Strobach H, Schrör K. Incomplete inhibition of platelet secretion by low-dose aspirin. Platelets . 1994;5(6):325–331. doi: 10.3109/09537109409006441. [DOI] [PubMed] [Google Scholar]
- 19.Parsa-Kondelaji M, Mansouritorghabeh H. Aspirin and clopidogrel resistance; a neglected gap in stroke and cardiovascular practice in Iran: a systematic review and meta-analysis. Thromb J . 2023;21(1):79. doi: 10.1186/s12959-023-00522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feher G, Feher A, Pusch G, Koltai K, Tibold A, Gasztonyi B, Papp E, Szapary L, Kesmarky G, Toth K. Clinical importance of aspirin and clopidogrel resistance. World J Cardiol . 2010;2(7):171–186. doi: 10.4330/wjc.v2.i7.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akturk IF, Caglar FN, Erturk M, Tuncer N, Yalcın AA, Surgit O, Uzun F, Caglar IM. Hypertension as a risk factor for aspirin and clopidogrel resistance in patients with stable coronary artery disease. Clin Appl Thromb Hemost . 2014;20(7):749–754. doi: 10.1177/1076029613481102. [DOI] [PubMed] [Google Scholar]
- 22.Salama MM, Morad AR, Saleh MA, Sabri NA, Zaki MM, ElSafady LA. Resistance to low-dose aspirin therapy among patients with acute coronary syndrome in relation to associated risk factors. J Clin Pharm Ther . 2012;37(6):630–636. doi: 10.1111/j.1365-2710.2009.01083.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen WH, Lee PY, Ng W, Tse HF, Lau CP. Aspirin resistance is associated with a high incidence of myonecrosis after non-urgent percutaneous coronary intervention despite clopidogrel pretreatment. J Am Coll Cardiol . 2004;43(6):1122–1126. doi: 10.1016/j.jacc.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Harris J, Pouwels KB, Johnson T, Sterne J, Pithara C, Mahadevan K, Reeves B, Benedetto U, Loke Y, Lasserson D, Doble B, Hopewell-Kelly N, Redwood S, Wordsworth S, Mumford A, Rogers C, Pufulete M. Bleeding risk in patients prescribed dual antiplatelet therapy and triple therapy after coronary interventions: the ADAPTT retrospective population-based cohort studies. Health Technol Assess . 2023;27(8):1–257. doi: 10.3310/MNJY9014. [DOI] [PMC free article] [PubMed] [Google Scholar]


