Abstract
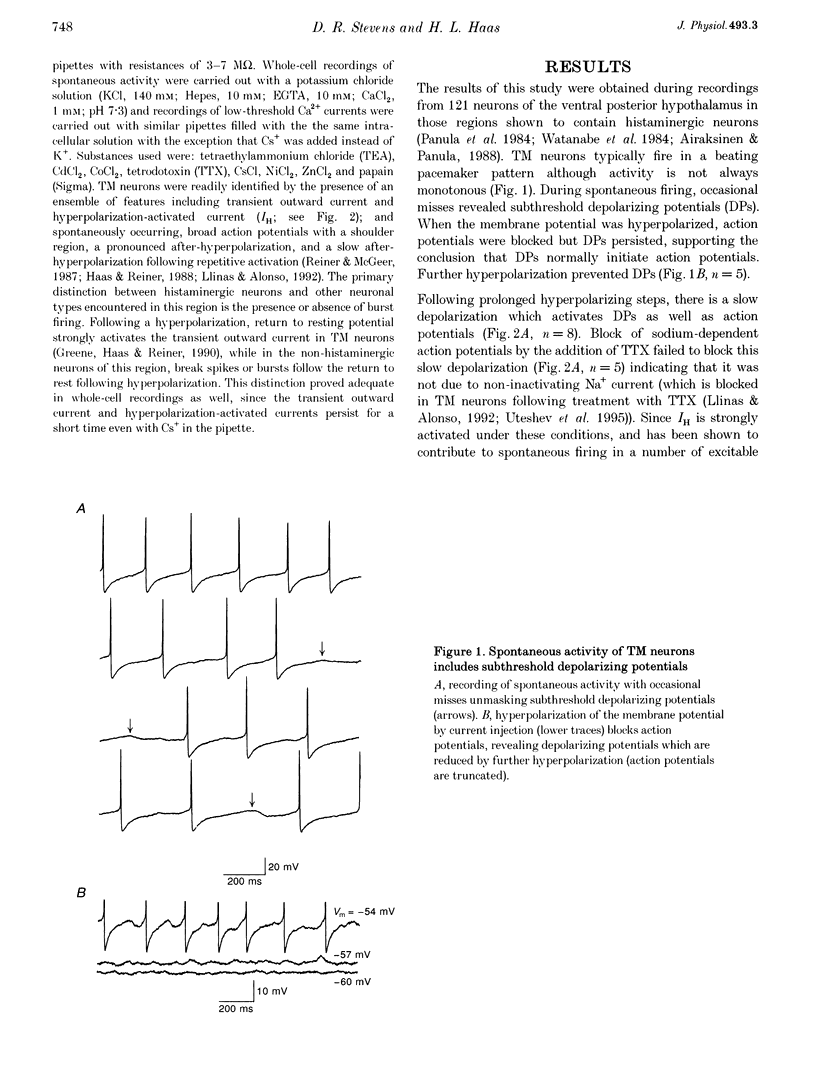
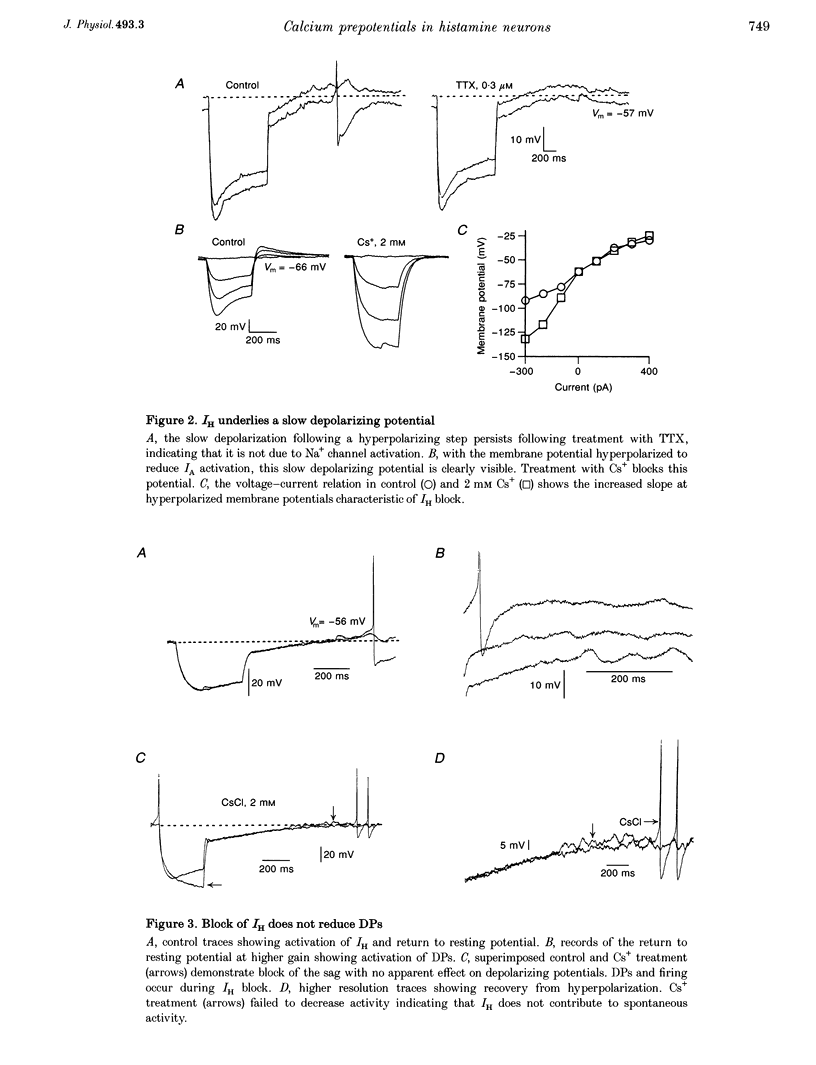
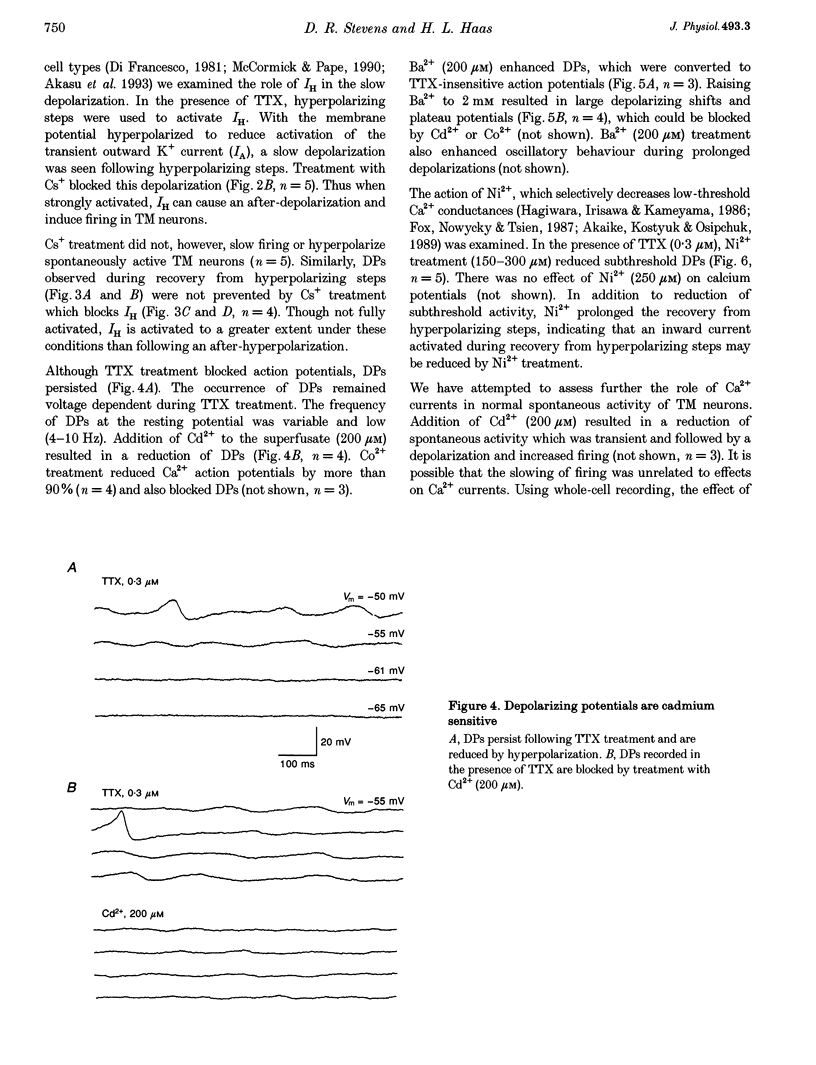
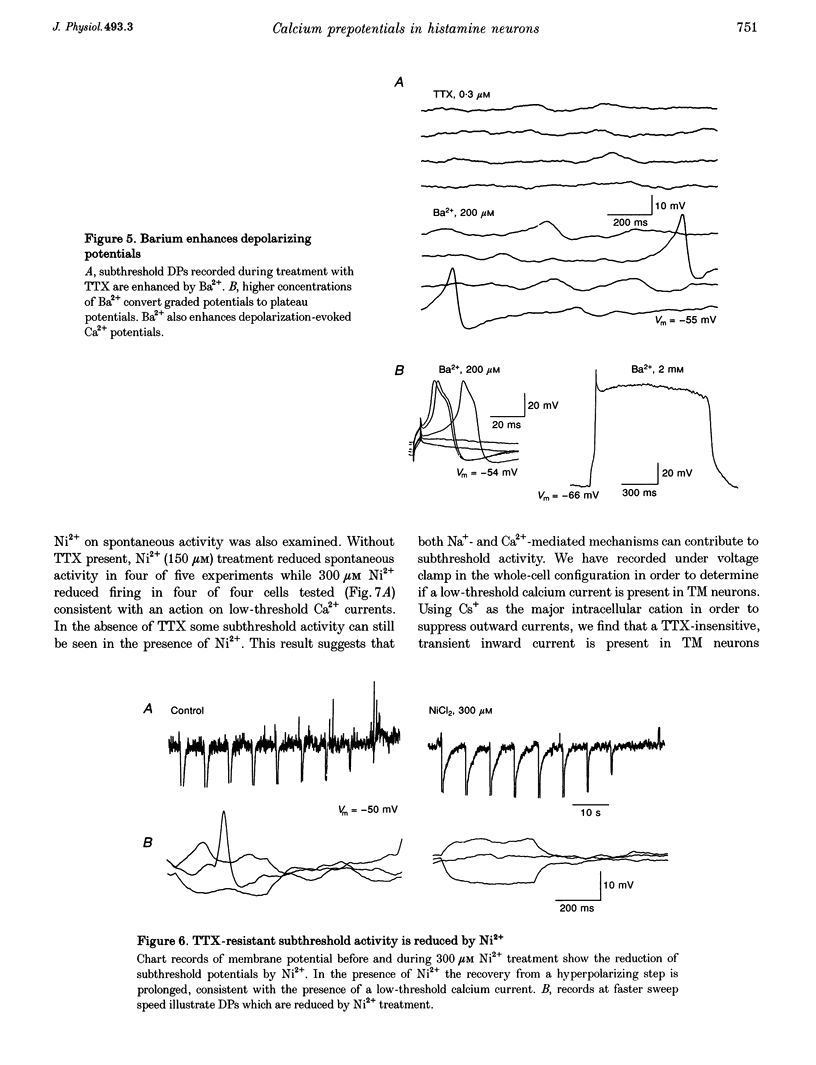
1. Intracellular recordings from histaminergic neurons of the tuberomammillary (TM) nucleus reveal subthreshold depolarizing potentials (DPs) which persist in the presence of tetrodotoxin. 2. Block of hyperpolarization-activated current by 1-4 mM Cs+ failed to reduce spontaneous activity or DPs. 3. In the presence of tetrodotoxin DPs are voltage dependent and are depressed by Cd2+ and Co2+. 4. Ba2+ (100 microM) treatment enhances DP amplitude and converts low-amplitude potentials to tetrodotoxin-insensitive action potentials. 5. In the presence of TTX, DPs are reduced by Ni2+. Spontaneous action potentials are also reduced by Ni2+ (100-300 microM). A low-threshold Ca2+ current is present which is sensitive to Ni2+. These results indicate the presence of calcium currents, perhaps of the low-threshold type, which contribute to activation of action potentials in TM neurons.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airaksinen M. S., Panula P. The histaminergic system in the guinea pig central nervous system: an immunocytochemical mapping study using an antiserum against histamine. J Comp Neurol. 1988 Jul 8;273(2):163–186. doi: 10.1002/cne.902730204. [DOI] [PubMed] [Google Scholar]
- Akaike N., Kostyuk P. G., Osipchuk Y. V. Dihydropyridine-sensitive low-threshold calcium channels in isolated rat hypothalamic neurones. J Physiol. 1989 May;412:181–195. doi: 10.1113/jphysiol.1989.sp017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Shoji S., Hasuo H. Inward rectifier and low-threshold calcium currents contribute to the spontaneous firing mechanism in neurons of the rat suprachiasmatic nucleus. Pflugers Arch. 1993 Oct;425(1-2):109–116. doi: 10.1007/BF00374510. [DOI] [PubMed] [Google Scholar]
- Alzheimer C., Schwindt P. C., Crill W. E. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci. 1993 Feb;13(2):660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlhis T. M., Aghajanian G. K. Pacemaker potentials of serotonergic dorsal raphe neurons: contribution of a low-threshold Ca2+ conductance. Synapse. 1987;1(6):582–588. doi: 10.1002/syn.890010611. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson H., Watanabe T., Köhler C. Morphological analysis of the tuberomammillary nucleus in the rat brain: delineation of subgroups with antibody against L-histidine decarboxylase as a marker. J Comp Neurol. 1987 Sep 1;263(1):1–24. doi: 10.1002/cne.902630102. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. A., Onn S. P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989 Oct;9(10):3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. W., Haas H. L., Reiner P. B. Two transient outward currents in histamine neurones of the rat hypothalamus in vitro. J Physiol. 1990 Jan;420:149–163. doi: 10.1113/jphysiol.1990.sp017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. L., Reiner P. B. Membrane properties of histaminergic tuberomammillary neurones of the rat hypothalamus in vitro. J Physiol. 1988 May;399:633–646. doi: 10.1113/jphysiol.1988.sp017100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. S., Sakai K., Vanni-Mercier G., Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989 Feb 13;479(2):225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- Senba E., Daddona P. E., Watanabe T., Wu J. Y., Nagy J. I. Coexistence of adenosine deaminase, histidine decarboxylase, and glutamate decarboxylase in hypothalamic neurons of the rat. J Neurosci. 1985 Dec;5(12):3393–3402. doi: 10.1523/JNEUROSCI.05-12-03393.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Crill W. E. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982 Mar 18;236(1):221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Uteshev V., Stevens D. R., Haas H. L. A persistent sodium current in acutely isolated histaminergic neurons from rat hypothalamus. Neuroscience. 1995 May;66(1):143–149. doi: 10.1016/0306-4522(94)00593-t. [DOI] [PubMed] [Google Scholar]
- Wada H., Inagaki N., Yamatodani A., Watanabe T. Is the histaminergic neuron system a regulatory center for whole-brain activity? Trends Neurosci. 1991 Sep;14(9):415–418. doi: 10.1016/0166-2236(91)90034-r. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Taguchi Y., Shiosaka S., Tanaka J., Kubota H., Terano Y., Tohyama M., Wada H. Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984 Mar 12;295(1):13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]


