ABSTRACT
How do mycobacteria divide? Cell division has been studied extensively in the model rod-shaped bacteria Escherichia coli and Bacillus subtilis, but much less is understood about cell division in mycobacteria, a genus that includes the major human pathogens M. tuberculosis and M. leprae. In general, bacterial cell division requires the concerted effort of many proteins in both space and time to elongate the cell, replicate and segregate the chromosome, and construct and destruct the septum - processes which result in the creation of two new daughter cells. Here, we describe these distinct stages of cell division in B. subtilis and follow with the current knowledge in mycobacteria. As will become apparent, there are many differences between mycobacteria and B. subtilis in terms of both the broad outline of cell division and the molecular details. So, while the fundamental challenge of spatially and temporally organizing cell division is shared between these rod-shaped bacteria, they have solved these challenges in often vastly different ways.
A toutes ces activités, il faut une cohésion rigoureuse pour que puisse se réaliser le rêve de la bactérie: produire deux bactéries. [To all these activities, it takes a rigorous cohesion to realize the dream of the bacterium: produce two bacteria.]
François Jacob, 1965 (1)
Bacterial psychology is a challenging field. Like many psychologists, Jacob impressed the bacterium with our own value system. And part of that is defining what it is to be alive. As microbiologists, we generally define bacterial life as the ability to form progeny, to become two bacteria through cell division. Like all reproductive processes, bacterial division uses a combination of mechanics and mystery. Here, we will focus on the mechanics (and mystery) of cell division in acid-fast mycobacteria, a genus that includes the major human pathogens Mycobacterium tuberculosis and Mycobacterium leprae.
From the 1968 study in which Hirota, Ryter, and Jacob classified temperature-sensitive mutants blocked for various steps of cell division in Escherichia coli (2) to the discovery of the FtsZ ring (Z-ring) in 1991 by Bi and Lutkenhaus, microbiologists have launched into an era of genetic, microscopic, biochemical, and structural work to understand bacterial cell growth and division (3). (For a review on the history of cell division research see reference 4). This topic has been studied most in the model rod-shaped bacteria, the Gram-negative E. coli and the Gram-positive Bacillus subtilis. Work on cell growth and division in mycobacteria is not as extensive as in E. coli or B. subtilis. However, mycobacteria face the same challenge as these other organisms: organizing, both spatially and temporally, the factors that are responsible for making two cells from one. This process requires the concerted effort of many molecules to elongate the cell, replicate and separate the chromosome, build the septal cell wall that separates the two daughter cells, and separate the now distinct daughter cells. As you will see in this article, one common theme in the nascent field of mycobacterial cell division is that mycobacteria have often solved these challenges differently, sometimes vastly so, than either E.coli or B. subtilis.
We will take a comparative approach. Throughout this article, we will describe distinct stages of cell division in B. subtilis and follow with the current knowledge of this process in mycobacteria. Mycobacteria are quite different than B. subtilis, and for many described proteins in B. subtilis, mycobacteria either lack homologs or use unique factors and protein interactions to fill these voids.
Cell elongation and division are intimately associated processes that require both synthesis and remodeling of the complex mycobacterial cell wall. The mycobacterial cell wall consists of covalently linked layers of fatty acids (mycolic acid), a carbohydrate polymer known as arabinogalactan, and an innermost layer of peptidoglycan (PG) (5). The envelope of most other bacteria, like B. subtilis, is comprised of PG, but these other polymers are unique to mycobacteria and related bacteria. We favor a model whereby the synthesis and remodeling of the distinct layers is coordinated, but there is currently little direct evidence to support this model.
MYCOBACTERIAL GROWTH: A DIFFERENT BALLGAME
The first obvious difference between B. subtilis and mycobacteria is in the geometry of cell division. To increase in size, mycobacteria insert new material at or near their poles, rather than along their side walls (Fig. 1) like B. subtilis and E. coli. However, where exactly cell wall synthesis occurs is still unclear. Many cell wall synthetic enzymes localize to the peri-polar region (6), but pulse chase experiments with dyes that covalently bind the cell wall suggest that new cell wall material is inserted at the very distal end of the cell (Fig. 2, bottom panel) (7). Supporting this last observation are labeling experiments with dyes that incorporate into different layers of the cell wall via enzymatic processes. These dyes brightly label the cell poles (8–10) (Fig. 2, top panel), as does a fluorescent analog of vancomycin (11, 12) which labels unprocessed, un-crosslinked PG. Thus, it seems most likely that mycobacteria insert a new cell wall at the distal ends of the poles. But how that is coordinated by enzymes that are most abundant at adjacent cellular regions will need to be reconciled in future research.
FIGURE 1.
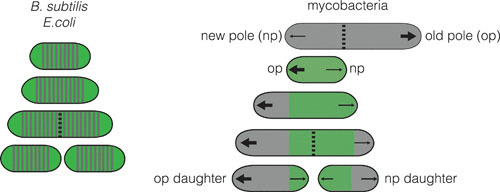
Characteristics of growth and division in B. subtilis and mycobacteria. B. subtilis and E. coli grow by adding new cell wall (gray) along the lateral cell body. Mycobacteria grow only at the polar regions, and do so at unequal amounts depending on the identity of the pole. This is observed by using a cell wall dye (green) to stain the existing cell wall and observe outgrowth of the newly synthesized, unstained cell wall (7). Arrows, polar location of new cell wall synthesis (a large arrow indicates more growth); dotted line, septum; green portion, old cell wall; gray portion, new cell wall.
FIGURE 2.
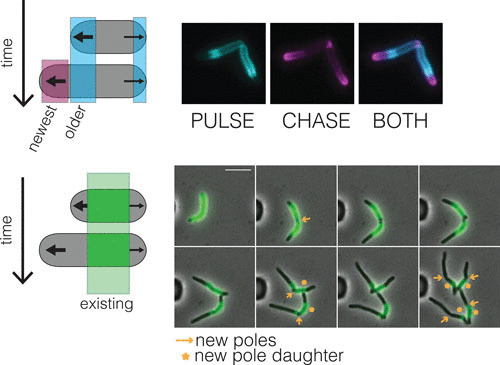
Polar growth segregates the cell wall based on age. (Top) Fluorescent d-amino acids are thought to incorporate into nascent PG. Pulse chase with these shows how the new and old cell walls are spatially segregated in M. smegmatis (Baranowski C, Rego EH, and Rubin EJ, unpublished images). (Bottom) Alexa-488 NHS ester stains the existing cell wall (green). New cell wall is unstained and can be monitored using time-lapse microscopy. After two divisions, the oldest cell wall is inherited by the new pole daughter cells (*) in M. smegmatis. (Baranowski, Rego, and Rubin. unpublished images; 7).
In general, polar growth leads to a different set of challenges not encountered by side-wall growers. First, the site of division in pole growers becomes the site of elongation. This means that division and elongation might need to be more tightly controlled temporally in mycobacteria than in B. subtilis, so that growth from the new poles does not occur before the cells completely divide. This raises a question: Are the components of the mycobacterial division complex (divisome) distinct from those of the elongation machinery (elongasome)? Throughout this review, you will see that mycobacterial cells blocked in certain points of division are elongated and form ectopic poles, suggesting that division and elongation can be uncoupled. This does not necessarily mean that the divisome and elongasome contain mutually exclusive proteins; however, it does support a model where division and elongation are temporally, and potentially spatially, separate. For example, there are localization, phenotypic, and protein-protein interaction data that place PonA1, a cell wall synthase, in both the elongasome and divisome. However, we lack a precise understanding of when the divisome becomes the elongasome at the new growth pole. Therefore, assigning proteins to either the divisome or elongasome based on localization at a single time point could be misleading, because factors involved in elongation also localize to the site of division/new pole and may belong to both machineries.
Second, polar growth leads to the spatial segregation of the cell wall with different ages, while lateral cell growth leads to the mixing of new and old material (7, 10, 13) (Fig. 2). In Mycobacterium smegmatis, it has been shown that the daughter cell inheriting the pole created at the most recent division, the so-called new pole, inherits the oldest cell wall (7) (Fig. 2, bottom panel). The functional consequence of this remains unknown, but it may contribute to the distinct characteristics of the daughter cells.
Lastly, the manner by which mycobacteria maintain their rod shape in the face of turgor pressure must be completely different than in the model rod-shaped organisms B. subtilis and E. coli. In B. subtilis, MreB, a homolog of eukaryotic actin, dictates the location of new cell wall synthesis along the side wall, incorporating new material at sites of negative curvature to aid in rod shape maintenance as the cell grows (14). This rod shape propagates in an MreB-dependent manner along the greatest membrane curvature (circumferentially around the cell) (15). Not only do mycobacteria lack an obvious homolog of MreB, but they grow from a much smaller portion of the cell (11). It remains a mystery how cell shape can be maintained across the entire cell. However, we do have a few clues. Mycobacteria encode a homolog of B. subtilis DivIVA called Wag31. In B. subtilis, depletion of DivIVA results in elongated cells that cannot divide, indicating a role in division (16). In contrast, depletion of Wag31 in mycobacteria leads to cells that lose their rod shape first at the pole, and eventually throughout the entire cell, resulting in a spherical cell. Further, dysregulation of Wag31 results in polar growth from incorrect sites (6). Thus, it appears that Wag31 in mycobacteria works to establish the site of polar growth, unlike its counterpart in B. subtilis, which has a role in dictating septum placement. Additionally, depletion of enzymes responsible for synthesizing or remodeling PG results in loss of rod shape either at the cell poles (17, 18) or along the mid-cell (6). Thus, it may be that Wag31 is responsible for directing formation of the poles and that the structure and rigidity of the cell wall itself, established at the time of cell wall synthesis at the pole, aids in shape maintenance as the cell grows.
THE MYSTERY AND MECHANICS OF DIVISION
Once a cell grows, it must divide to make two cells. The factors involved in cell division are referred to collectively as the divisome, and they work in concert at the mid-cell. The purpose of the divisome is to (i) identify the mid-cell for Z-ring placement; (ii) recruit Z-ring anchors, stabilizers, and regulators; (iii) build the septal cell wall; and (iv) split the two halves of the partitioned cell. The bacterial chromosome must be replicated and segregated in concert with cell division; however, this process will not be covered in this review.
Identify the Mid-Cell for Z-Ring Placement
FtsZ, a tubulin homolog, polymerizes into a ring (Z-ring) at the mid-cell to begin cell division (3; reviewed in 19). The mid-cell placement and stabilization of the Z-ring are the critical first stages of bacterial cell division. Recent work using metabolic labeling of PG, the crucial innermost cell wall layer, as well as fluorescent fusions to FtsZ and other well-characterized septal proteins in B. subtilis shows that FtsZ treadmilling directs septal synthesis in an inward-spiral fashion (you can also imagine a bullseye) (20). Thus, the Z-ring is the figurative and literal ringleader of bacterial cell division. Once placed, the Z-ring recruits a cascade of factors, both structural and enzymatic, required for synthesizing and splitting the septum (which becomes the new cell poles) to create two daughter cells (for review of FtsZ see references 19 and 21).
An (a)side on (a)symmetry
B. subtilis grows along the lateral cell body and divides at the mid-cell with striking precision (22), resulting in two nearly identical daughter cells. However, many observers (though not all) have observed that, on average, mycobacteria grow asymmetrically, with growth occurring in unequal amounts from the two poles (5). Multiple reports show that the old pole continues to grow during and after septation but that the new pole—the pole established at septation—takes some time before it grows. Thus, the overall amount of elongation from the poles in one cell cycle is different. This can easily be visualized by staining the existing cell wall with a dye and following the outgrowth of a new, unstained cell wall (6, 7, 23, 24). Likewise, the amount of incorporated PG-specific fluorescent d-amino acids supports this finding (10) (Fig. 2). While the total amount of growth appears different between the poles, the rate of cell wall addition may be the same between the poles at the end of division (10, 25, 26).
Besides potential differences in growth from the distinct poles, divisome placement may also be asymmetric. Fluorescent fusions to FtsZ show that septal placement in mycobacteria occurs over a range of the mid-cell, skewed toward the new pole (26–28). As a result of asymmetry in growth and Z-ring placement, mycobacterial daughter cells are phenotypically distinct—cells inheriting the new pole are smaller, while old-pole daughters are larger (5). The consequences and molecular details of this heterogeneity are poorly understood. Recently, however, a mycobacteria-specific protein, LamA (loss of asymmetry mutant A), was found to actively promote asymmetry by inhibiting growth at the new pole (23). Loss of LamA leads to a population of cells that can be more uniformly killed by certain antibiotics, suggesting that heterogeneity generated through cell growth and division confers a survival advantage in some conditions.
Identify the Mid-Cell for Z-Ring Placement (continued)
FtsZ is highly conserved in nearly all bacteria, including mycobacteria. One unique feature of mycobacterial FtsZ is that its polymerization can be inhibited, at least in vitro, via phosphorylation by the serine/threonine protein kinase, PknA (29, 30). In general, phosphorylation by PknA and PknB, both of which are essential for growth, plays a key role in regulating many mycobacterial divisome components. Depletion of either of these kinases results in elongated cells, implying a defect in division (31). Throughout this review, we will highlight specific ways in which division is regulated via phosphorylation by these kinases.
Where not to put the Z-ring: over the chromosome and at the poles (see also 32). Division occurring over the chromosome is lethal, and creation of a daughter cell without DNA is not useful. Therefore, mechanisms exist to prevent Z-ring formation over the chromosome and at the poles. B. subtilis has two systems that prevent Z-ring formation from occurring at these nonoptimal locations—Noc and Min. The Noc protein (nucleoid occlusion) binds along the chromosome, avoiding the replication terminus (which ends up orienting at mid-cell) (32). It then spreads from primary sites and associates with the membrane to inhibit Z-ring formation over the nucleoid (32). The Min system prevents the Z-ring from forming at the poles. The B. subtilis Min system comprises MinC,D,J and DivIVA. MinC is recruited and activated by membrane-bound MinD to interact with and inhibit FtsZ. This MinCD complex is recruited to the poles by the DivIVA interacting partner, MinJ (33). DivIVA is believed to localize to the septum/new pole late during division, at sites of negative membrane curvature, where it begins the recruitment of the Min system (34). To date, the mycobacterial DivIVA homolog, Wag31, has no described function in septal placement and, instead, functions in late septation/early pole establishment through interactions with non-Min-related septal and polar machinery.
Mycobacteria do not have a known Noc homolog, and there has been only one report of a single Min protein distantly related to MinD (Ssd). Cells overexpressing ssd are longer, and transcriptional profiling shows numerous genes involved in growth and division to be repressed (35). However, we do not yet know whether this protein acts in a system akin to B. subtilis Min.
While the exact molecular mechanism of septal placement in mycobacteria remains unknown, it appears that the mycobacterial cell wall again plays a role. A recent study utilizing atomic force microscopy identified troughs on the mycobacterial cell surface that formed at the poles and, eventually, became the sites of division one or two generations later. This suggests that potential Z-ring placement sites are created and inherited well before division has begun, the earliest described marker of Z-ring placement in bacteria to date. The nature of the troughs and the mechanism of their formation are not yet known, but this might represent a novel Z-ring placement strategy in bacteria (28).
Recruitment of Z-Ring Anchors, Stabilizers, and Regulators
FtsZ resides in the cytoplasm yet must trigger events occurring outside of the plasma membrane. Accordingly, is must be anchored to the membrane, an action achieved through various “early” interacting proteins. Once the Z-ring is anchored, the septal wall must be synthesized and daughter cells must then be split. Thus, the “late” divisome is comprised of structural proteins that recruit PG synthesis enzymes and these enzymes themselves. While in B. subtilis, there is clear distinction between the early and late divisome (∼20% of the cell cycle passes between recruitment of these portions of the complex), this distinction is less well defined in mycobacteria but only perhaps due to a lack of data (36).
Early divisome
The goal in the early steps of divisome assembly is to help the Z-ring polymerize and to anchor it to the membrane. The FtsZ ring is dynamic and requires both stabilizing and destabilizing proteins to function properly (21). Division in B. subtilis begins with Z-ring formation at the mid-cell, which recruits FtsA (37), SepF, ZapA, and EzrA (21). FtsA, an actin homolog, and SepF help anchor the Z-ring to the membrane (21). ZapA interacts with FtsZ and promotes its polymerization and stability (21, 32). A mutant of the transmembrane protein EzrA produces extra Z-rings (hence the name). The resultant mutant cells are longer, suggesting that negative regulation of the Z-ring by EzrA is necessary for successful division. This protein has been shown to inhibit Z-ring polymerization in vitro (21, 34). EzrA also works to recruit PBP1, a PG synthase, to the divisome (38).
Thus far, only SepF from B. subtilis’s early, FtsZ-interacting divisome has been identified in mycobacteria. B. subtilis SepF localizes to the septum in an FtsZ-dependent fashion and anchors and stabilizes the Z-ring (21, 39). In addition, in B. subtilis, sepF is synthetically lethal with ftsA and ezrA, perhaps due to overlapping functions (21). Likewise, mycobacterial SepF localizes to the septum in an FtsZ-dependent manner. Depletion and overexpression of sepF leads to filaments and branching—canonical phenotypes of cell division inhibition in mycobacteria (39).
In B. subtilis, ClpX, the ATPase chaperone/substrate recognition portion of the ClpXP protease, inhibits FtsZ assembly. Intriguingly, this appears to be independent of its ATPase function (40, 41). Similarly, mycobacterial ClpX interacts directly (in vivo) and inhibits FtsZ polymerization (in vitro). Overexpression of ClpX leads to multinucleate, elongated cells, suggesting a defect in septal assembly and division (42).
Connecting the cytoplasmic steps to the periplasmic steps
After the early B. subtilis divisome is assembled (the Z-ring anchored and stabilized), “late” divisome proteins are recruited (43). These proteins connect the cytoplasmic steps of division to the periplasmic steps required to build the septal cell wall (reviewed in 21). To summarize decades of work briefly, a complex of structural proteins—FtsQ (DivIB), FtsL, and FtsB (DivIC), transmembrane proteins that are critical in connecting Z-ring formation to septal synthesis—and FtsW, a PG transglycosylase, localize to the septum to recruit other PG synthases. The timing and dependency of septal protein localization in B. subtilis reveals interdependency between members of the FtsQLB complex, FtsW and Pbp2B (a monofunctional PG transpeptidase) (36). This is in contrast to the more linear dependency of localization in E. coli: FtsK→FtsQLB→FtsW→PBP3 (34).
Mycobacterial homologs of the essential ftsL and ftsB have been identified and experimentally validated through localization, depletion, and in silico structural predictions (44). Intriguingly, these homologs have substantial N- and C-terminal expansions compared to their B. subtilis counterparts, presumably for mycobacteria-specific interaction partners. In mycobacteria, depletion of either ftsL or ftsB causes cells to filament and branch, a phenocopy of known septal protein depletions (ftsZ, pbpB). FtsL and B, along with FtsQ and FtsK (a DNA translocase), localize to the septum. Depletion studies allow inferred localization dependencies of these structural divisome components in mycobacteria. Localization of FtsK, FtsQ, FtsL, or FtsB depend upon FtsZ. FtsK localization does not require FtsQ, L, or B. FtsQ can partially localize in the depletion of FtsL or FtsB, supporting a preliminary dependency hierarchy: FtsZ→FtsK→FtsQ→FtsLB (44). Thus, finding counterparts to known B. subtilis divisome members provides a handle to identify and study previously uncharacterized mycobacteria-specific divisome proteins (Fig. 3, brown dashed lines).
FIGURE 3.
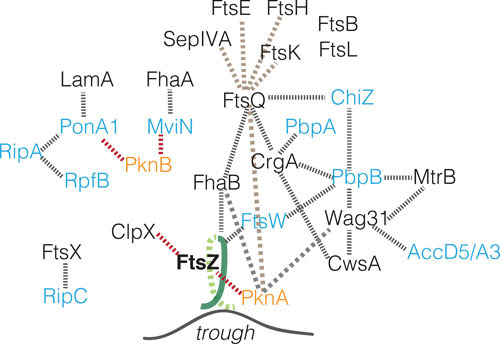
Mycobacterial divisome interactions. A schematic of mycobacterial divisome protein interactions. Note that interactions are not necessarily direct given the available data. Gray dotted lines, physical interactions; red dotted lines, negative regulation; brown lines, FtsQ pulldown proteins (44); blue text, cell wall enzymes; orange text, kinases.
One potential mycobacterial “work-around” in the absence of canonical Z-ring anchors like FtsA is the unique interaction between mycobacterial FtsZ and FtsW. FtsW is the divisome-specific homolog of the SEDS (shape, elongation, division, sporulation) family protein RodA, whose function is to polymerize glycan strands of PG through its transglycosylase function (45). Mycobacterial FtsW and FtsZ have noncanonical C-terminal extensions that are proposed to facilitate their interaction (46). They localize at the septum in a manner suggesting that FtsZ localizes before FtsW (47). FtsW also interacts with and appears to recruit PbpB, a monofunctional PG transpeptidase, to the septum (48). In cells depleted of FtsW, FtsZ localizes between nucleoids, but these cells are not proficient at division (48). By interacting with early divisome-founding FtsZ and late divisome septal PG synthase PbpB, FtsW appears to link beginning cytoplasmic steps with late periplasmic wall synthesis steps in mycobacteria.
In actinomycetes, the cascade of interactions is facilitated by the small transmembrane protein, CrgA. CrgA is involved in nearly all steps of cell division: from the start of division, to septal wall synthesis, to establishment of the new growth poles. CrgA interacts with FtsZ and two PG transpeptidases, PbpA and PbpB. It localizes to the septum after the Z-ring and may be required for proper recruitment of PbpB, similarly to FtsW (49). CrgA also interacts with the mycobacteria-specific CwsA, a transmembrane protein that interacts with Wag31 (50). Expression of cwsA is directly linked to Wag31 abundance at the poles, suggesting that it is an important factor in either recruiting or maintaining Wag31 at the poles (50). Since Wag31 is associated with new polar growth, the information flow from CrgA through CwsA to Wag31 may coordinate establishing the new pole at the site of division.
Another transmembrane protein, FhaB (also called FipA), interacts with FtsZ. This relationship depends on phosphorylation of FhaB by PknA. FhaB is required for interactions between FtsZ and FtsQ during oxidative stress, a condition that pathogenic mycobacteria experience in the host, suggesting that it may be a signal transducing interaction between the Z-ring and the structural divisome protein FtsQ during intracellular division (5, 30).
While these examples are understood in some detail, several other proteins produce morphologic changes when disrupted. Loss of proteins such as the whiB2 homolog whmD and the MinD like protein Ssd result in branching and chaining morphologies, phenotypes associated with disrupted cell division (35, 51). The functions of these proteins remain unknown.
Call to Arms: Septal Cell Wall Synthetic Machinery To Build a Wall
Constructing the septal wall
Division requires synthesis of a wall between two halves of the cell and physical separation of the two halves into independent daughter cells. The cell wall of mycobacteria is multifaceted, consisting of linked layers of PG, arabinogalactan, and mycolic acids (5). The coordination between layers seems likely, but evidence of this is currently slim. This discussion of septal cell wall synthesis will focus on the PG layer.
PG, a netlike structure comprising disaccharide chains cross-linked by peptide bridges, is located outside of the plasma membrane. PG synthesis begins in the cytoplasm, where the disaccharide backbone and pentapeptide side chain are linked to a lipid and flipped across the membrane by the lipidII flippase, MurJ (MviN in mycobacteria) (52) (Fig. 4). This unit is added to the existing glycan strand by the transglycosylase function of bifunctional penicillin-binding proteins (class A PBPs or aPBPs) and by SEDS proteins such as RodA (45, 53). To covalently close the PG network into a cage-like molecule, the peptide bridges are cross-linked between either the fourth and third (4-3) or the third and third (3-3) residues of opposite PG strands. The 4-3 cross-links, those commonly found in most well-studied PG, are catalyzed by the transpeptidation function of aPBPs and/or by mono-functional class B PBPs (bPBPs), which possess only transpeptidase activity (53). The 3-3 cross-links, a variety of connection that is rare in the PG of other model rods but highly enriched in mycobacteria, are made by l,d-transpeptidases (5, 54, 55) (Fig. 4).
FIGURE 4.
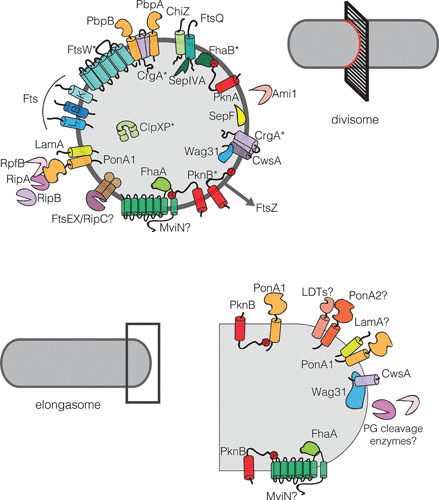
Mycobacterial divisome and elongasome members. (Top) Schematic of proteins involved in mycobacterial cell division. Proteins marked with an asterisk (*) have been shown to interact with FtsZ. The FtsZ ring is illustrated as a dark gray circle upon which the divisome members are arranged. (Bottom) Schematic of proteins involved in mycobacterial elongation. Interacting proteins are depicted touching, and proteins with a question mark (?) may belong in these complexes, but data are limited.
Mycobacteria encode homologs of the B. subtilis proteins that perform the essential cytoplasmic steps of PG synthesis, but there are important differences in their regulation. For example, the first committed step of PG synthesis, performed in the cytoplasm by MurA, is regulated by a CwlM, a degenerate PG amidase homolog (56). Phosphorylation of CwlM by PknB results in binding and activation of MurA to initiate PG synthesis. This regulatory cascade functions in times of starvation when CwlM is dephosphorylated, resulting in a halt of PG synthesis and reductive cell division.
Another difference is MviN, which is also regulated via phosphorylation in mycobacteria. MviN’s phosphorylation by PknB recruits FhaA to a pseudokinase domain of MviN. Depletion of MviN causes cells to arrest PG synthesis as measured by precursor abundance in the cytoplasm. FhaA depletion leads to an accumulation of PG illustrated with a fluorescent derivative of vancomycin. FhaA localizes to the poles and septum. Together, these data suggest that FhaA may work to inhibit PG synthesis at the MviN step. Alternatively, FhaA may be required for a PG processing event required to remove the substrate for fluorescent vancomycin binding (57).
After lipid II is flipped across the plasma membrane, it must be incorporated into the existing PG network by both transglyosylases and transpeptidases. In B. subtilis, it is possible to delete all of the aPBPs, suggesting the presence of another glycosyltransferase (58). In B. subtilis, this was recently identified to be RodA in the elongation complex (45) and is likely FtsW, a homolog of RodA, in the division complex. In mycobacteria, the story again appears slightly different. PonA1, an aPBP, is an essential member of the M. smegmatis elongation complex and is synthetically lethal with PonA2, another aPBP, in M. tuberculosis (59). PonA1 is essential because of its transglycosylase activity (24). RodA is not annotated essential in either organism. On the other hand, FtsW is essential and localizes to the site of division. Thus, these data suggest that, in contrast to B. subtilis, the bifunctional PBPs are the major transglycosylases involved in elongation in mycobacteria. During division, the story may be more similar to B. subtilis, with FtsW fulfilling the major transglycosylase role.
Working in concert with the PG transglycosylases are transpeptidases. Again, this role can be filled by either aPBPs or bPBPs. There are two main divisome-associated bPBPs in B. subtilis, Pbp1 and Pbp2B. Pbp2B (also known as FtsI or PbpB in mycobacteria) localizes to the septum in an FtsQLB-dependent manner (60) and works in partnership with FtsW (21, 36). Pbp1 is thought to shuttle in a cell cycle dependent between old septa (poles), to the sidewall, and then to the division complex via interactions with GspB and EzrA. (38, 61).
In mycobacteria, there is evidence for both an aPBP (PonA1) and bPBPs (PbpB, PbpA) being involved in division. PbpB interacts with divisome members FtsW, MtrB, and CrgA (see above; Fig. 3). Depletion of pbpB leads to branching, filamenting cells, clearly blocked in cell division (44, 62). Cells lacking pbpA are longer than the wild type, and PbpA may localize to the septum (63). While PonA1 plays a critical role in elongation, it may also have a role during division. The best evidence for this is that PonA1 localizes to both the septum and poles and interacts with RipA, a PG hydrolase important for daughter cell separation (64). However, given the difficulty of assigning proteins to the division or elongation complex based on protein localization at a single time point (see “Mycobacterial Growth: a Different Ballgame”), it could be that PonA1’s appearance at the septum reflects recruitment to the site of the nascent poles. Indeed, genetic modulation of PonA1’s function results in elongation defects rather than canonical cell division defects (24). Further, its overexpression results in the appearance of atopic poles, suggesting an early role in the establishment of polar elongation.
Breaking the cell wall
Once the wall is made between the two halves of the cell,it must be split to create two independent daughter cells. This is achieved in B. subtilis through hydrolysis of PG by LytC, D, E, and F (65–67). Localization studies found LytE and LytF of B. subtilis at the septum and poles (68).
Currently, three PG hydrolases have been implicated in mycobacterial cell division: RipA/B, ChiZ, and Ami1. RipA and B are operonic d,l-endopeptidases that cleave within PG peptide side chains (69). These proteins have been shown to be synthetically lethal with one another (70). RipA interacts with resuscitation promoting factor B (RpfB), a lytic transglycosylase, as well as with PonA1. It is cleaved and activated by the MarP protease, specifically in acidic conditions like those found in host phagosomes (10). RipA localizes to the poles and the septum (64, 71). Cells depleted of the ripAB operon chain and branch (septa are made but not cleaved), suggesting that this operon is required for daughter cell separation. RipA PG cleavage is synergistic with RpfB (72). Intriguingly, RipA and PonA1 interact in the same domain as RipA/RpfB. Adding PonA1 to a mixture of RipA and RpfB reduces the rate PG hydrolysis by the RipA/RpfB complex but does not inhibit RipA activity alone, suggesting a that the interaction of RipA with PonA1 and RpfB helps coordinate the synthesis and breakdown of the septal wall (5, 17).
ChiZ, like many PG hydrolases, contains a LysM domain known to bind PG (73). ChiZ shares some similarities with B. subtilis YneA, a protein whose expression is induced by DNA damage, which curbs division by inhibiting the Z-ring and cell separation (74, 75). Overexpression of yneA leads to filaments, but intriguingly, these filaments can be bypassed by a divIB (ftsQ) mutation further linking YneA with cell division (75). Similarly, overexpression of chiZ leads to filamentation, and Z-ring assembly appears partially defective; some normal Z-rings appear to form, but much of FtsZ appears distributed throughout the cell. ChiZ does not appear to inhibit FtsZ polymerization in vitro (74). It interacts with PbpB and FtsQ but does not appear to affect their localization (76). The mechanism by which ChiZ and YneA act in division remains unclear.
Ami1, a putative PG amidase, was recently found to be involved in mycobacterial division. In Δami1 cells, division is blocked, leading to filamentation and lateral/polar branching. Stability of Z-rings appears compromised in these cells, and lysis at the site of attempted septation is observed (77).
Notably, PG hydrolysis is a dangerous game for a bacterial cell, so hydrolase enzymes must be carefully regulated. FtsEX, an ABC transporter complex, is required for proper cell division in E. coli through recruitment of EnvC, a regulator of PG amidases at the septum and also through interaction with early divisome anchor FtsA (78, 79). However, FtsEX appears to regulate the CwlO hydrolase during cell elongation and not division in B. subtilis (80). In mycobacteria it has been shown that FtsX interacts with a RipA homolog, RipC, but the role of these proteins in division is yet to be explored (81).
Physical separation of daughter cells in B. subtilis occurs slowly. In contrast, mycobacterial cell separation has been described as “V-snapping,” where daughter cells literally snap away from each other at the septum but often retain a point of contact (like the quick opening of a door) (82). High-speed imaging has recently revealed that actinobacteria, like mycobacteria, split rapidly, in approximately 10 milliseconds (83). While some mycobacterial cells V-snap, some cells quickly push away from each other at the septum but fail to V-snap; rather, they “straight” snap. This is likely due to the complexity of the mycobacterial cell wall, which must be quickly sheared for full V-snapping (83). In fact, all layers of the cell wall, including PG, arabinogalactan, and mycolic acids, are present at the site of the septum by metabolic labeling (8, 10).
THE CIRCLE OF LIFE: CREATING A NEW GROWTH POLE
In B. subtilis, the late septum, post-separation, becomes a synthesis-inert pole. In stark contrast, the pole created at division in mycobacteria becomes the location of cell wall synthesis, the hub of growth for these bacteria.
As previously mentioned, B. subtilis DivIVA is a cooperative partner of the Min system to prevent septation over the nucleoid. This can be classified as an early division inhibitor at the stage of Z-ring placement. Conversely, the mycobacterial DivIVA homologue, known as Wag31 or Antigen84, plays a critical role in late stage septation/new pole establishment. It is essential for survival, and its misregulation results in distinct division and elongation impairment phenotypes—branching cells in wag31 overexpression (84) and polar bulges when depleted (6, 12). Like B. subtilis DivIVA, the N-terminus appears to direct Wag31 to polar curvature that may help it localize to the poles (6). Wag31 is phosphorylated by PknA/B (31, 85), but it is unclear how phosphorylation regulates its function (62, 85).
Wag31 localizes primarily to the old pole (i.e., the pole that existed before the most recent division [Fig. 1]) and is observed at the very late septum after the membrane has come down and separated daughter cells (12, 26). This postmembrane septal localization may indicate a role for Wag31 in the transition from a septum to a pole (26). In line with this, Wag31 interacts with members of both division (septal PG synthase PbpB (62, 86), MtrB-discussed below) and elongation (CwsA) machinery. The interaction of Wag31 and PbpB prevents protease cleavage of PbpB in oxidative stress conditions such as growth in macrophages. This highlights a critical link between in vivo stresses and cell division (86).
In addition, Wag31 has been shown to interact with the mycolic acid synthesis enzymes AccA3 and AccD5 (6), supporting the notion of coordination between the mycobacterial layers. Interestingly, these enzymes localize in a manner like Wag31, primarily at the old pole but also at the transitioning septum (6), again suggesting that Wag31 may be involved in the creation of new poles at the site of division.
Wag31 is not an enzyme, and yet, it must somehow coordinate cell wall synthases to the pole. How this is accomplished is unknown. We do know that one major PG synthase involved in elongating the cells, PonA1, is also regulated by phosphorylation in mycobacteria. PonA1 is phosphorylated by PknB, an essential Ser/Thr eukaryotic-like kinase (24). A phosphoablative mutation in PonA1 increases the rate of cell elongation. Thus, PonA1’s activity is negatively regulated by phosphorylation (24). PonA1 further interacts, either directly or indirectly, with LamA, a mycobacteria-specific protein that actively inhibits growth from the new pole to create asymmetry in polar growth, at the late divisome (23) (see “An (a)side on (a)symmetry” above). Like FtsZ ring assembly, there may be both stimulatory and repressive forces at play to maintain balance in cell wall synthesis.
MYCOBACTERIAL GROWTH RATE: DOES SLOW AND STEADY WIN THE RACE?
Many laboratory bacteria are famous for their ability to rapidly multiply. Mycobacteria, however, grow and divide relatively slowly or, in some cases, not at all under laboratory conditions. There is diversity even among the mycobacterial genus. For example, soil-dwelling M. smegmatis are “fast growers,” dividing once every ∼2.5 hours. On the other side of the spectrum are the human pathogens M. tuberculosis and M. leprae, which grow extremely slowly during infection (between 20 hours to a few days for M. tuberculosis and ∼2 weeks for M. leprae (87)). Cultured M. tuberculosis grows at a similar rate (∼20 hours) (88), suggesting that slow growth is not simply a matter of host restriction. Why do mycobacteria grow slowly? The short answer is that we do not know, but that will not stop us from speculating.
One possibility is that polar growth leads to a different set of rules governing bacterial growth. At what point does a bacterium divide? Is it based on time, on size, or on amount of growth? These three hypotheses are known as the sizer, the timer, and the adder models, respectively. Recent work supports the idea that bacteria such as E. coli, Caulobacter crescentus, and B. subtilis may adhere to the adder model, whereby cells add a constant amount of volume during a cell cycle, independent of cell size (89, 90).
The adder model relies on exponential growth. In lateral growers such as B. subtilis this is an easy feat to accomplish: as new cell wall is inserted along the entirety of the cell body, the area of new addition also grows. Polar growth, however, does not so simply allow for exponential addition because expansion is restrained to a specific, and proportionally small, geographic area of the cell. Indeed, at first it seemed that mycobacteria might be governed by a different principle. Early work suggested that mycobacteria employ a timer mechanism, dividing after a certain amount of time independent of cell size (7). However, two recent studies strongly suggest that mycobacteria are governed by the same adder principle as other bacteria, albeit with modifications (91, 92). One surprising conclusion from these studies is that single mycobacterial cells appear to grow exponentially (26, 92).
Another possible factor in slow growth is the formidable nutrient barrier provided by the mycobacterial cell wall. And, of course, there is a strong causal relationship between nutrient availability and growth rate: in nutrient-rich environments, bacteria grow faster and, to an extent, become larger. Is the availability of nutrients rate-limiting in mycobacteria? M. smegmatis, the most well-studied fast-growing mycobacteria, encodes a porin, MspA, which aids in the uptake of nutrients across the myco-membrane. Notably, MspA is absent in M. tuberculosis (93). Expressing mspA in BCG, a slow-growing mycobacteria naturally lacking mspA, caused the cells to slightly but reproducibly increase their doubling rate, supporting a link between nutrient uptake and growth rate in mycobacteria (93–95). Likewise, porin knockdowns in the fast-growing mycobacteria Mycobacterium fortuitum, lead to a decrease in colony size, suggesting that nutrient uptake plays some role in growth rate (96). However, this must only be part of the story, because expression of MspA only marginally increased the growth rate of BCG.
An additional putative factor in the slow growth of mycobacteria is the rate of DNA replication. In many other species, the demands of fast growth necessitate mutlifork DNA replication because DNA replication is slower than the process of growth and division in nutrient-rich conditions (97). Until recently, it was thought that mycobacteria were not capable of multifork DNA replication, thus decreasing the potential to increase the growth rate beyond this rate-limiting step. However, a recent study challenges this idea, showing multifork replication using time-lapse microscopy and fluorescent reporters of replication-related proteins (98). Intriguingly, the authors found that multifork replication occurred in nutrient-rich and nutrient-poor conditions. Thus, it remains unclear what role multifork replication plays in mycobacteria. Perhaps mycobacterial DNA polymerization is inherently slow. M. smegmatis has an estimated DNA synthesis rate of 400 bases/second. This is quite rapid compared to M. tuberculosis with a proposed rate of 50 bases/second (98, 99). However, these rates are sluggish in relation to the 600 to 1,000 bases/second that E. coli and B. subtilis can accomplish (100). DNA replication, the so-called “C” period of the cell cycle, takes up ∼75% of the M. smegmatis cell cycle (26, 98). Oddly, the in vitro rate of nucleotide incorporation by mycobacterial DNA polymerase DnaE1 is faster than that of E. coli PolIIIα (101). This suggests that conditions within the cell, for example, limited nucleotide pools, restrict the rate of new chromosomal synthesis (100).
Yet another possibility is that the construction of the intricate cell wall itself limits the growth rate of mycobacteria. Supporting this idea is the recent E. coli study by Vadia et al. (102). They proposed an “outside-in” model, in which E. coli cell size is limited by fatty-acid synthesis so that cell volume does not increase at a rate faster than can be accommodated by the cell envelope. Certainly, cell wall fatty acid synthesis could be a contributing factor to mycobacterial slow growth since mycobacteria must synthesize their complex and long mycolic acids, and there are substantial cell wall differences between fast- and slow-growing mycobacteria (93).
MORE DEEP THOUGHTS ON MYCOBACTERIAL DIVISION
While bacteria dream of dividing, pathogens dream of dividing within the host. And mycobacteria seem to be exquisitely tuned for doing so: M. tuberculosis is an obligate pathogen with essentially no environmental niche. And it is highly successful. In 2016 there were over 10 million new cases of M. tuberculosis infection estimated and nearly 2 million deaths (103). Thus, an important part of thinking about the growth of this organism is to consider its adaptations to the host. The slow growth rate is likely optimized for surviving in humans, though why this should be remains unclear. And the ability to survive for decades as an asymptomatic infection, perhaps growing slowly or not at all, permits a reservoir of infection that ensures the long-term survival of the species. The cell wall is appropriately adapted to interface with the host (5). And while the pattern of growth has been inherited from the soil-dwelling organisms from which it has likely evolved, M. tuberculosis takes full advantage of it to avoid clearance during infection.
The outcome of these adaptations is crucial when we consider therapy for tuberculosis. The thick cell wall and its multiple embedded efflux pumps present a formidable barrier for many drugs that are effective against other bacteria (104). At the same time, it creates new opportunities to target structures and processes that are absent in model organisms. For example, antibiotics such as ethambutol and isoniazid that inhibit the formation of arabinogalactan and mycolic acid synthesis, respectively, are absolute mainstays of tuberculosis therapy. With a better understanding of the underlying molecular mechanisms, other unique aspects of mycobacterial cell division could provide effective and specific targets for tuberculosis therapy.
Those of us who study mycobacteria are indebted to the groundbreaking research conducted in B. subtilis and E. coli—work that has created a template on which to base our thinking about our own organisms. But mycobacteria create their own, unique paradigms. Model bacteria might dream alike, but each unusual bacterium dreams in its own way. C’est la vie!
Contributor Information
Catherine Baranowski, Department of Immunology and Infectious Disease, Harvard T. H. Chan School of Public Health, Boston, MA 02115.
E. Hesper Rego, Department of Microbial Pathogenesis, Yale University School of Medicine, New Haven, CT 06510.
Eric J. Rubin, Department of Immunology and Infectious Disease, Harvard T. H. Chan School of Public Health, Boston, MA 02115 Department of Microbiology and Immunobiology, Harvard Medical School, Boston, MA 02115.
Vincent A. Fischetti, The Rockefeller University, New York, NY
Richard P. Novick, Skirball Institute for Molecular Medicine, NYU Medical Center, New York, NY
Joseph J. Ferretti, Department of Microbiology & Immunology, University of Oklahoma Health Science Center, Oklahoma City, OK
Daniel A. Portnoy, Department of Molecular and Cellular Microbiology, University of California, Berkeley, Berkeley, CA
Julian I. Rood, Australian Bacterial Pathogen Program, Department of Microbiology, Monash University, Melbourne, Australia
REFERENCES
- 1.Jacob F. 1965. Leçon inaugurale: faite le vendredi 7 mai 1965 (Collège de France).
- 2.Hirota Y, Ryter A, Jacob F. 1968. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol 33:677–693 10.1101/SQB.1968.033.01.077. [PubMed] 10.1101/SQB.1968.033.01.077 [DOI] [PubMed] [Google Scholar]
- 3.Bi EF, Lutkenhaus J. 1991. FtsZ ring structure associated with division in Escherichia coli. Nature 354:161–164 10.1038/354161a0. [PubMed] 10.1038/354161a0 [DOI] [PubMed] [Google Scholar]
- 4.Blaauwen den T, Hamoen LW, Levin PA. 2017. The divisome at 25: the road ahead. Curr Opin Microbiol 36:85–94. [PubMed] 10.1016/j.mib.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kieser KJ, Rubin EJ. 2014. How sisters grow apart: mycobacterial growth and division. Nat Rev Microbiol 12:550–562 10.1038/nrmicro3299. [PubMed] 10.1038/nrmicro3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meniche X, Otten R, Siegrist MS, Baer CE, Murphy KC, Bertozzi CR, Sassetti CM. 2014. Subpolar addition of new cell wall is directed by DivIVA in mycobacteria. Proc Natl Acad Sci U S A 111:E3243–E3251 10.1073/pnas.1402158111. [PubMed] 10.1073/pnas.1402158111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aldridge BB, Fernandez-Suarez M, Heller D, Ambravaneswaran V, Irimia D, Toner M, Fortune SM. 2012. Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science 335:100–104 10.1126/science.1216166. 10.1126/science.1216166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley HN, Stewart JA, Kavunja HW, Rundell SR, Swarts BM. 2016. Bioorthogonal chemical reporters for selective in situ probing of mycomembrane components in mycobacteria. Angew Chem Int Ed Engl 55:2053–2057 10.1002/anie.201509216. 10.1002/anie.201509216 [DOI] [PubMed] [Google Scholar]
- 9.Siegrist MS, Whiteside S, Jewett JC, Aditham A, Cava F, Bertozzi CR. 2013. (D)-amino acid chemical reporters reveal peptidoglycan dynamics of an intracellular pathogen. ACS Chem Biol 8:500–505 10.1021/cb3004995. 10.1021/cb3004995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botella H, Yang G, Ouerfelli O, Ehrt S, Nathan CF, Vaubourgeix J. 2017. Distinct spatiotemporal dynamics of peptidoglycan synthesis between Mycobacterium smegmatis and Mycobacterium tuberculosis. MBio 8:e01183-e17 10.1128/mBio.01183-17. 10.1128/mBio.01183-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel RA, Errington J. 2003. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113:767–776 10.1016/S0092-8674(03)00421-5. [PubMed] 10.1016/S0092-8674(03)00421-5 [DOI] [PubMed] [Google Scholar]
- 12.Kang C-M, Nyayapathy S, Lee J-Y, Suh J-W, Husson RN. 2008. Wag31, a homologue of the cell division protein DivIVA, regulates growth, morphology and polar cell wall synthesis in mycobacteria. Microbiology 154:725–735 10.1099/mic.0.2007/014076-0. 10.1099/mic.0.2007/014076-0 [DOI] [PubMed] [Google Scholar]
- 13.Cameron TA, Zupan JR, Zambryski PC. 2015. The essential features and modes of bacterial polar growth. Trends Microbiol 23:347–353 10.1016/j.tim.2015.01.003. 10.1016/j.tim.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 14.Ursell TS, Nguyen J, Monds RD, Colavin A, Billings G, Ouzounov N, Gitai Z, Shaevitz JW, Huang KC. 2014. Rod-like bacterial shape is maintained by feedback between cell curvature and cytoskeletal localization. Proc Natl Acad Sci U S A 111:E1025–E1034 10.1073/pnas.1317174111. 10.1073/pnas.1317174111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussain S, Wivagg CN, Szwedziak P, Wong F, Schaefer K, Izore T, Renner LD, Sun Y, Bisson Filho AW, Walker S, Amir A, Löwe J, Garner EC. 2017. MreB filaments create rod shape by aligning along principal membrane curvature. BioRxiv https://www.biorxiv.org/content/early/2017/10/02/197475.1. 10.1101/197475 [DOI]
- 16.Edwards DH, Errington J. 1997. The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol Microbiol 24:905–915 10.1046/j.1365-2958.1997.3811764.x. 10.1046/j.1365-2958.1997.3811764.x [DOI] [PubMed] [Google Scholar]
- 17.Hett EC, Chao MC, Rubin EJ. 2010. Interaction and modulation of two antagonistic cell wall enzymes of mycobacteria. PLoS Pathog 6:e1001020-14 10.1371/journal.ppat.1001020. [PubMed] 10.1371/journal.ppat.1001020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders AN, Wright LF, Pavelka MS Jr. 2014. Genetic characterization of mycobacterial l,d-transpeptidases. Microbiology 160:1795–1806 10.1099/mic.0.078980-0. [PubMed] 10.1099/mic.0.078980-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haeusser DP, Margolin W. 2016. Splitsville: structural and functional insights into the dynamic bacterial Z ring. Nat Rev Microbiol 14:305–319 10.1038/nrmicro.2016.26. [PubMed] 10.1038/nrmicro.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisson-Filho AW, Hsu Y-P, Squyres GR, Kuru E, Wu F, Jukes C, Sun Y, Dekker C, Holden S, VanNieuwenhze MS, Brun YV, Garner EC. 2017. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science 355:739–743 10.1126/science.aak9973. [PubMed] 10.1126/science.aak9973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams DW, Errington J. 2009. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol 7:642–653 10.1038/nrmicro2198. [PubMed] 10.1038/nrmicro2198 [DOI] [PubMed] [Google Scholar]
- 22.Migocki MD, Freeman MK, Wake RG, Harry EJ. 2002. The Min system is not required for precise placement of the midcell Z ring in Bacillus subtilis. EMBO Rep 3:1163–1167 10.1093/embo-reports/kvf233. [PubMed] 10.1093/embo-reports/kvf233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rego EH, Audette RE, Rubin EJ. 2017. Deletion of a mycobacterial divisome factor collapses single-cell phenotypic heterogeneity. Nature 546:153–157 10.1038/nature22361. [PubMed] 10.1038/nature22361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieser KJ, Boutte CC, Kester JC, Baer CE, Barczak AK, Meniche X, Chao MC, Rego EH, Sassetti CM, Fortune SM, Rubin EJ. 2015. Phosphorylation of the peptidoglycan synthase PonA1 governs the rate of polar elongation in mycobacteria. PLoS Pathog 11:e1005010 10.1371/journal.ppat.1005010. [PubMed] 10.1371/journal.ppat.1005010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakamoto Y, Dhar N, Chait R, Schneider K, Signorino-Gelo F, Leibler S, McKinney JD. 2013. Dynamic persistence of antibiotic-stressed mycobacteria. Science 339:91–95 10.1126/science.1229858. [PubMed] 10.1126/science.1229858 [DOI] [PubMed] [Google Scholar]
- 26.Santi I, Dhar N, Bousbaine D, Wakamoto Y, McKinney JD. 2013. Single-cell dynamics of the chromosome replication and cell division cycles in mycobacteria. Nat Commun 4:2470 10.1038/ncomms3470. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Joyce G, Williams KJ, Robb M, Noens E, Tizzano B, Shahrezaei V, Robertson BD. 2012. Cell division site placement and asymmetric growth in mycobacteria. PLoS One 7:e44582 10.1371/journal.pone.0044582. [PubMed] 10.1371/journal.pone.0044582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eskandarian HA, Odermatt PD, Ven JXY, Hannebelle MTM, Nievergelt AP, Dhar N, McKinney JD, Fantner GE. 2017. Division site selection linked to inherited cell surface wave troughs in mycobacteria. Nat Microbiol 2:17094 10.1038/nmicrobiol.2017.94. [PubMed] 10.1038/nmicrobiol.2017.94 [DOI] [PubMed] [Google Scholar]
- 29.Thakur M, Chakraborti PK. 2006. GTPase activity of mycobacterial FtsZ is impaired due to its transphosphorylation by the eukaryotic-type Ser/Thr kinase, PknA. J Biol Chem 281:40107–40113 10.1074/jbc.M607216200. [PubMed] 10.1074/jbc.M607216200 [DOI] [PubMed] [Google Scholar]
- 30.Sureka K, Hossain T, Mukherjee P, Chatterjee P, Datta P, Kundu M, Basu J. 2010. Novel role of phosphorylation-dependent interaction between FtsZ and FipA in mycobacterial cell division. PLoS One 5:e8590 10.1371/journal.pone.0008590. [PubMed] 10.1371/journal.pone.0008590 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Kang C-M, Abbott DW, Park ST, Dascher CC, Cantley LC, Husson RN. 2005. The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev 19:1692–1704 10.1101/gad.1311105. 10.1101/gad.1311105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu LJ, Errington J. 2011. Nucleoid occlusion and bacterial cell division. Nat Rev Microbiol 10:8–12 10.1038/nrmicro2671. [PubMed] 10.1038/nrmicro2671 [DOI] [PubMed] [Google Scholar]
- 33.Patrick JE, Kearns DB. 2008. MinJ (YvjD) is a topological determinant of cell division in Bacillus subtilis. Mol Microbiol 70:1166–1179 10.1111/j.1365-2958.2008.06469.x. [PubMed] 10.1111/j.1365-2958.2008.06469.x [DOI] [PubMed] [Google Scholar]
- 34.Egan AJF, Vollmer W. 2013. The physiology of bacterial cell division. Ann N Y Acad Sci 1277:8–28 10.1111/j.1749-6632.2012.06818.x. [PubMed] 10.1111/j.1749-6632.2012.06818.x [DOI] [PubMed] [Google Scholar]
- 35.England K, Crew R, Slayden RA. 2011. Mycobacterium tuberculosis septum site determining protein, Ssd encoded by rv3660c, promotes filamentation and elicits an alternative metabolic and dormancy stress response. BMC Microbiol 11:79 10.1186/1471-2180-11-79. [PubMed] 10.1186/1471-2180-11-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gamba P, Hamoen LW, Daniel RA. 2016. Cooperative recruitment of FtsW to the division site of Bacillus subtilis. Front Microbiol 7:1808 10.3389/fmicb.2016.01808. [PubMed] 10.3389/fmicb.2016.01808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feucht A, Lucet I, Yudkin MD, Errington J. 2001. Cytological and biochemical characterization of the FtsA cell division protein of Bacillus subtilis. Mol Microbiol 40:115–125 10.1046/j.1365-2958.2001.02356.x. 10.1046/j.1365-2958.2001.02356.x [DOI] [PubMed] [Google Scholar]
- 38.Claessen D, Emmins R, Hamoen LW, Daniel RA, Errington J, Edwards DH. 2008. Control of the cell elongation-division cycle by shuttling of PBP1 protein in Bacillus subtilis. Mol Microbiol 68:1029–1046 10.1111/j.1365-2958.2008.06210.x. [PubMed] 10.1111/j.1365-2958.2008.06210.x [DOI] [PubMed] [Google Scholar]
- 39.Gola S, Munder T, Casonato S, Manganelli R, Vicente M. 2015. The essential role of SepF in mycobacterial division. Mol Microbiol 97:560–576 10.1111/mmi.13050. [PubMed] 10.1111/mmi.13050 [DOI] [PubMed] [Google Scholar]
- 40.Haeusser DP, Lee AH, Weart RB, Levin PA. 2009. ClpX inhibits FtsZ assembly in a manner that does not require its ATP hydrolysis-dependent chaperone activity. J Bacteriol 191:1986–1991 10.1128/JB.01606-07. 10.1128/JB.01606-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weart RB, Nakano S, Lane BE, Zuber P, Levin PA. 2005. The ClpX chaperone modulates assembly of the tubulin-like protein FtsZ. Mol Microbiol 57:238–249 10.1111/j.1365-2958.2005.04673.x. [PubMed] 10.1111/j.1365-2958.2005.04673.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dziedzic R, Kiran M, Plocinski P, Ziolkiewicz M, Brzostek A, Moomey M, Vadrevu IS, Dziadek J, Madiraju M, Rajagopalan M. 2010. Mycobacterium tuberculosis ClpX interacts with FtsZ and interferes with FtsZ assembly. PLoS One 5:e11058 10.1371/journal.pone.0011058. [PubMed] 10.1371/journal.pone.0011058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gamba P, Veening JW, Saunders NJ, Hamoen LW, Daniel RA. 2009. Two-step assembly dynamics of the Bacillus subtilis divisome. J Bacteriol 191:4186–4194 10.1128/JB.01758-08. [PubMed] 10.1128/JB.01758-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu KJ, Zhang J, Baranowski C, Leung V, Rego EH, Morita Y, Rubin EJ, Boutte CC. 2018. Characterization of conserved and novel septal factors in Mycobacterium smegmatis. J Bacteriol 200:e00649-17. [PubMed] 10.1128/JB.00649-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meeske AJ, Riley EP, Robins WP, Uehara T, Mekalanos JJ, Kahne D, Walker S, Kruse AC, Bernhardt TG, Rudner DZ. 2016. SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature 537:634–638 10.1038/nature19331. [PubMed] 10.1038/nature19331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Datta P, Dasgupta A, Bhakta S, Basu J. 2002. Interaction between FtsZ and FtsW of Mycobacterium tuberculosis. J Biol Chem 277:24983–24987 10.1074/jbc.M203847200. [PubMed] 10.1074/jbc.M203847200 [DOI] [PubMed] [Google Scholar]
- 47.Rajagopalan M, Maloney E, Dziadek J, Poplawska M, Lofton H, Chauhan A, Madiraju MVVS. 2005. Genetic evidence that mycobacterial FtsZ and FtsW proteins interact, and colocalize to the division site in Mycobacterium smegmatis. FEMS Microbiol Lett 250:9–17 10.1016/j.femsle.2005.06.043. 10.1016/j.femsle.2005.06.043 [DOI] [PubMed] [Google Scholar]
- 48.Datta P, Dasgupta A, Singh AK, Mukherjee P, Kundu M, Basu J. 2006. Interaction between FtsW and penicillin-binding protein 3 (PBP3) directs PBP3 to mid-cell, controls cell septation and mediates the formation of a trimeric complex involving FtsZ, FtsW and PBP3 in mycobacteria. Mol Microbiol 62:1655–1673 10.1111/j.1365-2958.2006.05491.x. 10.1111/j.1365-2958.2006.05491.x [DOI] [PubMed] [Google Scholar]
- 49.Plocinski P, Ziolkiewicz M, Kiran M, Vadrevu SI, Nguyen HB, Hugonnet J, Veckerle C, Arthur M, Dziadek J, Cross TA, Madiraju M, Rajagopalan M. 2011. Characterization of CrgA, a new partner of the Mycobacterium tuberculosis peptidoglycan polymerization complexes. J Bacteriol 193:3246–3256 10.1128/JB.00188-11. 10.1128/JB.00188-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plocinski P, Arora N, Sarva K, Blaszczyk E, Qin H, Das N, Plocinska R, Ziolkiewicz M, Dziadek J, Kiran M, Gorla P, Cross TA, Madiraju M, Rajagopalan M. 2012. Mycobacterium tuberculosis CwsA interacts with CrgA and Wag31, and the CrgA-CwsA complex is involved in peptidoglycan synthesis and cell shape determination. J Bacteriol 194:6398–6409 10.1128/JB.01005-12. 10.1128/JB.01005-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez JE, Bishai WR. 2000. whmD is an essential mycobacterial gene required for proper septation and cell division. Proc Natl Acad Sci U S A 97:8554–8559 10.1073/pnas.140225297. [PubMed] 10.1073/pnas.140225297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sham L-T, Butler EK, Lebar MD, Kahne D, Bernhardt TG, Ruiz N. 2014. Bacterial cell wall. MurJ is the flippase of lipid-linked precursors for peptidoglycan biogenesis. Science 345:220–222 10.1126/science.1254522. 10.1126/science.1254522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Typas A, Banzhaf M, Gross CA, Vollmer W. 2011. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol 10:123–136 10.1038/nrmicro2677. 10.1038/nrmicro2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavollay M, Arthur M, Fourgeaud M, Dubost L, Marie A, Veziris N, Blanot D, Gutmann L, Mainardi JL. 2008. The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by l,d-transpeptidation. J Bacteriol 190:4360–4366 10.1128/JB.00239-08. 10.1128/JB.00239-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar P, Arora K, Lloyd JR, Lee IY, Nair V, Fischer E, Boshoff HIM, Barry CE III. 2012. Meropenem inhibits d,d-carboxypeptidase activity in Mycobacterium tuberculosis. Mol Microbiol 86:367–381 10.1111/j.1365-2958.2012.08199.x. 10.1111/j.1365-2958.2012.08199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boutte CC, Baer CE, Papavinasasundaram K, Liu W, Chase MR, Meniche X, Fortune SM, Sassetti CM, Ioerger TR, Rubin EJ. 2016. A cytoplasmic peptidoglycan amidase homologue controls mycobacterial cell wall synthesis. eLife 5:a021113 10.7554/eLife.14590. 10.7554/eLife.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gee CL, Papavinasasundaram KG, Blair SR, Baer CE, Falick AM, King DS, Griffin JE, Venghatakrishnan H, Zukauskas A, Wei J-R, Dhiman RK, Crick DC, Rubin EJ, Sassetti CM, Alber T. 2012. A phosphorylated pseudokinase complex controls cell wall synthesis in mycobacteria. Sci Signal 5:ra7 10.1126/scisignal.2002525. [PubMed] 10.1126/scisignal.2002525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meeske AJ, Sham L-T, Kimsey H, Koo B-M, Gross CA, Bernhardt TG, Rudner DZ. 2015. MurJ and a novel lipid II flippase are required for cell wall biogenesis in Bacillus subtilis. Proc Natl Acad Sci U S A 112:6437–6442 10.1073/pnas.1504967112. [PubMed] 10.1073/pnas.1504967112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kieser KJ, Baranowski C, Chao MC, Long JE, Sassetti CM, Waldor MK, Sacchettini JC, Ioerger TR, Rubin EJ. 2015. Peptidoglycan synthesis in Mycobacterium tuberculosis is organized into networks with varying drug susceptibility. Proc Natl Acad Sci U S A 112:13087–13092 10.1073/pnas.1514135112. 10.1073/pnas.1514135112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donovan C, Bramkamp M. 2014. Cell division in Corynebacterineae. Front Microbiol 5:132 10.3389/fmicb.2014.00132. [PubMed] 10.3389/fmicb.2014.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tavares JR, de Souza RF, Meira GLS, Gueiros-Filho FJ. 2008. Cytological characterization of YpsB, a novel component of the Bacillus subtilis divisome. J Bacteriol 190:7096–7107 10.1128/JB.00064-08. 10.1128/JB.00064-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plocinska R, Martinez L, Gorla P, Pandeeti E, Sarva K, Blaszczyk E, Dziadek J, Madiraju MV, Rajagopalan M. 2014. Mycobacterium tuberculosis MtrB sensor kinase interactions with FtsI and Wag31 proteins reveal a role for MtrB distinct from that regulating MtrA activities. J Bacteriol 196:4120–4129 10.1128/JB.01795-14. 10.1128/JB.01795-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dasgupta A, Datta P, Kundu M, Basu J. 2006. The serine/threonine kinase PknB of Mycobacterium tuberculosis phosphorylates PBPA, a penicillin-binding protein required for cell division. Microbiology 152:493–504 10.1099/mic.0.28630-0. [PubMed] 10.1099/mic.0.28630-0 [DOI] [PubMed] [Google Scholar]
- 64.Chao MC, Kieser KJ, Minami S, Mavrici D, Aldridge BB, Fortune SM, Alber T, Rubin EJ. 2013. Protein complexes and proteolytic activation of the cell wall hydrolase RipA regulate septal resolution in mycobacteria. PLoS Pathog 9:e1003197 10.1371/journal.ppat.1003197. [PubMed] 10.1371/journal.ppat.1003197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith TJ, Blackman SA, Foster SJ. 2000. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology 146:249–262 10.1099/00221287-146-2-249. 10.1099/00221287-146-2-249 [DOI] [PubMed] [Google Scholar]
- 66.Ohnishi R, Ishikawa S, Sekiguchi J. 1999. Peptidoglycan hydrolase LytF plays a role in cell separation with CwlF during vegetative growth of Bacillus subtilis. J Bacteriol 181:3178–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ishikawa S, Hara Y, Ohnishi R, Sekiguchi J. 1998. Regulation of a new cell wall hydrolase gene, cwlF, which affects cell separation in Bacillus subtilis. J Bacteriol 180:2549–2555. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheffers D-J, Pinho MG. 2005. Bacterial cell wall synthesis: new insights from localization studies. Microbiol Mol Biol Rev 69:585–607 10.1128/MMBR.69.4.585-607.2005. [PubMed] 10.1128/MMBR.69.4.585-607.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Böth D, Schneider G, Schnell R. 2011. Peptidoglycan remodeling in Mycobacterium tuberculosis: comparison of structures and catalytic activities of RipA and RipB. J Mol Biol 413:247–260 10.1016/j.jmb.2011.08.014. 10.1016/j.jmb.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 70.Martinelli DJ, Pavelka MS Jr. 2016. The RipA and RipB peptidoglycan endopeptidases are individually nonessential to Mycobacterium smegmatis. J Bacteriol 198:1464–1475 10.1128/JB.00059-16. 10.1128/JB.00059-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hett EC, Chao MC, Steyn AJ, Fortune SM, Deng LL, Rubin EJ. 2007. A partner for the resuscitation-promoting factors of Mycobacterium tuberculosis. Mol Microbiol 66:658–668 10.1111/j.1365-2958.2007.05945.x. 10.1111/j.1365-2958.2007.05945.x [DOI] [PubMed] [Google Scholar]
- 72.Hett EC, Chao MC, Deng LL, Rubin EJ. 2008. A mycobacterial enzyme essential for cell division synergizes with resuscitation-promoting factor. PLoS Pathog 4:e1000001 10.1371/journal.ppat.1000001. [PubMed] 10.1371/journal.ppat.1000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buist G, Steen A, Kok J, Kuipers OP. 2008. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol 68:838–847 10.1111/j.1365-2958.2008.06211.x. 10.1111/j.1365-2958.2008.06211.x [DOI] [PubMed] [Google Scholar]
- 74.Chauhan A, Lofton H, Maloney E, Moore J, Fol M, Madiraju MVVS, Rajagopalan M. 2006. Interference of Mycobacterium tuberculosis cell division by Rv2719c, a cell wall hydrolase. Mol Microbiol 62:132–147 10.1111/j.1365-2958.2006.05333.x. 10.1111/j.1365-2958.2006.05333.x [DOI] [PubMed] [Google Scholar]
- 75.Mo AH, Burkholder WF. 2010. YneA, an SOS-induced inhibitor of cell division in Bacillus subtilis, is regulated posttranslationally and requires the transmembrane region for activity. J Bacteriol 192:3159–3173 10.1128/JB.00027-10. 10.1128/JB.00027-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vadrevu IS, Lofton H, Sarva K, Blasczyk E, Plocinska R, Chinnaswamy J, Madiraju M, Rajagopalan M. 2011. ChiZ levels modulate cell division process in mycobacteria. Tuberculosis (Edinb) 91(Suppl 1):S128–S135 10.1016/j.tube.2011.10.022. 10.1016/j.tube.2011.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Senzani S, Li D, Bhaskar A, Ealand C, Chang J, Rimal B, Liu C, Joon Kim S, Dhar N, Kana B. 2017. An Amidase_3 domain-containing N-acetylmuramyl-l-alanine amidase is required for mycobacterial cell division. Sci Rep 7:1140 10.1038/s41598-017-01184-7. [PubMed] 10.1038/s41598-017-01184-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du S, Pichoff S, Lutkenhaus J. 2016. FtsEX acts on FtsA to regulate divisome assembly and activity. Proc Natl Acad Sci U S A 113:E5052–E5061 10.1073/pnas.1606656113. [PubMed] 10.1073/pnas.1606656113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang DC, Peters NT, Parzych KR, Uehara T, Markovski M, Bernhardt TG. 2011. An ATP-binding cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. Proc Natl Acad Sci U S A 108:E1052–E1060 10.1073/pnas.1107780108. 10.1073/pnas.1107780108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meisner J, Montero Llopis P, Sham L-T, Garner E, Bernhardt TG, Rudner DZ. 2013. FtsEX is required for CwlO peptidoglycan hydrolase activity during cell wall elongation in Bacillus subtilis. Mol Microbiol 89:1069–1083 10.1111/mmi.12330. 10.1111/mmi.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mavrici D, Marakalala MJ, Holton JM, Prigozhin DM, Gee CL, Zhang YJ, Rubin EJ, Alber T. 2014. Mycobacterium tuberculosis FtsX extracellular domain activates the peptidoglycan hydrolase, RipC. Proc Natl Acad Sci U S A 111:8037–8042 10.1073/pnas.1321812111. 10.1073/pnas.1321812111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thanky NR, Young DB, Robertson BD. 2007. Unusual features of the cell cycle in mycobacteria: polar-restricted growth and the snapping-model of cell division. Tuberculosis (Edinb) 87:231–236 10.1016/j.tube.2006.10.004. 10.1016/j.tube.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 83.Zhou X, Halladin DK, Theriot JA. 2016. Fast mechanically driven daughter cell separation is widespread in actinobacteria. MBio 7:e00952-16 10.1128/mBio.00952-16. [PubMed] 10.1128/mBio.00952-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen L, Scherr N, Gatfield J, Walburger A, Pieters J, Thompson CJ. 2007. Antigen 84, an effector of pleiomorphism in Mycobacterium smegmatis. J Bacteriol 189:7896–7910 10.1128/JB.00726-07. [PubMed] 10.1128/JB.00726-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jani C, Eoh H, Lee JJ, Hamasha K, Sahana MB, Han J-S, Nyayapathy S, Lee J-Y, Suh J-W, Lee SH, Rehse SJ, Crick DC, Kang C-M. 2010. Regulation of polar peptidoglycan biosynthesis by Wag31 phosphorylation in mycobacteria. BMC Microbiol 10:327 10.1186/1471-2180-10-327. [PubMed] 10.1186/1471-2180-10-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mukherjee P, Sureka K, Datta P, Hossain T, Barik S, Das KP, Kundu M, Basu J. 2009. Novel role of Wag31 in protection of mycobacteria under oxidative stress. Mol Microbiol 73:103–119 10.1111/j.1365-2958.2009.06750.x. [PubMed] 10.1111/j.1365-2958.2009.06750.x [DOI] [PubMed] [Google Scholar]
- 87.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honoré N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. 2001. Massive gene decay in the leprosy bacillus. Nature 409:1007–1011 10.1038/35059006. 10.1038/35059006 [DOI] [PubMed] [Google Scholar]
- 88.Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. 2009. A replication clock for Mycobacterium tuberculosis. Nat Med 15:211–214 10.1038/nm.1915. [PubMed] 10.1038/nm.1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Campos M, Surovtsev IV, Kato S, Paintdakhi A, Beltran B, Ebmeier SE, Jacobs-Wagner C. 2014. A constant size extension drives bacterial cell size homeostasis. Cell 159:1433–1446 10.1016/j.cell.2014.11.022. [PubMed] 10.1016/j.cell.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taheri-Araghi S, Bradde S, Sauls JT, Hill NS, Levin PA, Paulsson J, Vergassola M, Jun S. 2015. Cell-size control and homeostasis in bacteria. Curr Biol 25:385–391 10.1016/j.cub.2014.12.009. [PubMed] 10.1016/j.cub.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Priestman M, Thomas P, Robertson BD, Shahrezaei V. 2017. Mycobacteria modify their cell size control under sub-optimal carbon sources. Front Cell Dev Biol 5:64 10.3389/fcell.2017.00064. [PubMed] 10.3389/fcell.2017.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Logsdon MM, Ho P-Y, Papavinasasundaram K, Richardson K, Cokol M, Sassetti CM, Amir A, Aldridge BB. 2017. A parallel adder coordinates mycobacterial cell-cycle progression and cell-size homeostasis in the context of asymmetric growth and organization. Curr Biol 27:3367–3374.e7 10.1016/j.cub.2017.09.046. [PubMed] 10.1016/j.cub.2017.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hett EC, Rubin EJ. 2008. Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev 72:126–156 10.1128/MMBR.00028-07. 10.1128/MMBR.00028-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharbati-Tehrani S, Meister B, Appel B, Lewin A. 2004. The porin MspA from Mycobacterium smegmatis improves growth of Mycobacterium bovis BCG. Int J Med Microbiol 294:235–245 10.1016/j.ijmm.2004.02.001. 10.1016/j.ijmm.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 95.Mailaender C, Reiling N, Engelhardt H, Bossmann S, Ehlers S, Niederweis M. 2004. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology 150:853–864 10.1099/mic.0.26902-0. 10.1099/mic.0.26902-0 [DOI] [PubMed] [Google Scholar]
- 96.Sharbati S, Schramm K, Rempel S, Wang H, Andrich R, Tykiel V, Kunisch R, Lewin A. 2009. Characterisation of porin genes from Mycobacterium fortuitum and their impact on growth. BMC Microbiol 9:31 10.1186/1471-2180-9-31. [PubMed] 10.1186/1471-2180-9-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang JD, Levin PA. 2009. Metabolism, cell growth and the bacterial cell cycle. Nat Rev Microbiol 7:822–827 10.1038/nrmicro2202. [PubMed] 10.1038/nrmicro2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trojanowski D, Ginda K, Pióro M, Hołówka J, Skut P, Jakimowicz D, Zakrzewska-Czerwińska J. 2015. Choreography of the Mycobacterium replication machinery during the cell cycle. MBio 6:e02125-14 10.1128/mBio.02125-14. [PubMed] 10.1128/mBio.02125-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nair N, Dziedzic R, Greendyke R, Muniruzzaman S, Rajagopalan M, Madiraju MV. 2009. Synchronous replication initiation in novel Mycobacterium tuberculosis dnaA cold-sensitive mutants. Mol Microbiol 71:291–304 10.1111/j.1365-2958.2008.06523.x. [PubMed] 10.1111/j.1365-2958.2008.06523.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ditse Z, Lamers MH, Warner DF. 2017. DNA Replication in Mycobacterium tuberculosis. Microbiol Spectr 5:TBTB2-0027-2016 10.1128/microbiolspec.TBTB2-0027-2016. 10.1128/microbiolspec.TBTB2-0027-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rock JM, Lang UF, Chase MR, Ford CB, Gerrick ER, Gawande R, Coscolla M, Gagneux S, Fortune SM, Lamers MH. 2015. DNA replication fidelity in Mycobacterium tuberculosis is mediated by an ancestral prokaryotic proofreader. Nat Genet 47:677–681 10.1038/ng.3269. 10.1038/ng.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vadia S, Tse JL, Lucena R, Yang Z, Kellogg DR, Wang JD, Levin PA. 2017. Fatty acid availability sets cell envelope capacity and dictates microbial cell size. Curr Biol 27:1757–1767.e5 10.1016/j.cub.2017.05.076. [PubMed] 10.1016/j.cub.2017.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.WHO. 2017. Global Tuberculosis Report 2017. WHO, Geneva, Switzerland. [Google Scholar]
- 104.Pule CM, Sampson SL, Warren RM, Black PA, van Helden PD, Victor TC, Louw GE. 2016. Efflux pump inhibitors: targeting mycobacterial efflux systems to enhance TB therapy. J Antimicrob Chemother 71:17–26 10.1093/jac/dkv316. [PubMed] 10.1093/jac/dkv316 [DOI] [PubMed] [Google Scholar]


