FIGURE 2.
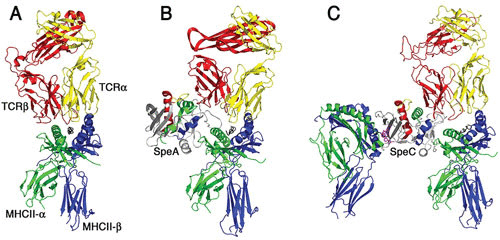
Models of T cell activation complexes for streptococcal superantigens. Ribbon diagrams demonstrating typical antigen-mediated T cell activation (A) and modeled T cell activation complexes for SpeA (B) and SpeC (C). The cocrystal structures of SpeA and SpeC in complex with their respective TCR β-chains (48) and of SpeC in complex with the MHC class II through the zinc-dependent high-affinity binding domain have been determined (169). SpeC also activates T cells in a mode similar to the staphylococcal enterotoxin A model (58) where SpeC engages MHC class II α-chain through a generic low-affinity binding domain (170) and engages the MHC class II β-chain through a zinc-dependent, high-affinity binding domain (169). The binding architecture for the generic low-affinity MHC class II binding to SpeA and SpeC is modeled using the staphylococcal enterotoxin B-MHC class II cocrystal structure (171). Note the presence of the zinc ion (magenta) coordinated in the high-affinity binding site for SpeC and that SpeA lacks this zinc site. The TCR α-chain (shown in gray) for both the SpeA and SpeC diagrams is modeled for clarity by superimposition of the α/β TCR shown on the left of the respective TCR β-chains for both superantigens. The figure was generated using Pymol.
