Abstract
Background
Currently, Vonoprazan (VPZ) and amoxicillin dual regimen (VA-dual) has not achieved satisfied efficacy as the first-line treatment for Helicobacter pylori (H. pylori) infection in China. Thus, we aimed to determine the effect of VA-dual plus Saccharomyces boulardii (S. boulardii) on H. pylori eradication rate.
Methods
Naive H. pylori-infected patients were randomly allocated to the ECAB group [20-mg esomeprazole, 500-mg clarithromycin, 1000-mg amoxicillin, and 220-mg bismuth twice/day for 14 days] or the VAS group [20-mg VPZ twice/day, 750-mg amoxicillin three times/day, and 250-mg S. boulardii twice/day for 10 days]. Factors associated with eradication success were explored, and cost-effectiveness analyses were also performed.
Results
Herein, 126 patients were finally included and randomly assigned to the two groups in a 1:1 ratio. The H. pylori eradication rates of VAS and ECAB groups by intention-to-treat analysis were 87.3% and 88.9% (P = 1.000) and by per-protocol analysis were 87.3% and 91.8% (P = 0.560), respectively. The ECAB group had a significantly higher incidence of adverse events than the VAS group. Superior H. pylori eradication in the VAS group was related to small body surface area and being a non-smoker. The cost-effectiveness ratio of the VAS group was less than that of the ECAB group.
Conclusions
Addition of S. boulardii to VA-dual for 10 days is as effective as the 14-days bismuth-based quadruple regimen while ensuring fewer adverse events and lesser cost. This regimen is particularly suitable for low-BSA patients or non-smokers.
Trial registration
Chinese Clinical trial Registry No. ChiCTR2100055101 31/12/2021.
Keywords: Vonoprazan, Saccharomyces Boulardii, H. pylori eradication, Cost-effectiveness
Introduction
It is estimated that approximately 50% of the world’s population has encountered Helicobacter pylori (H. pylori) infection, thus leading to its recognition as a public health threat worldwide [1]. H. pylori often causes gastrointestinal complications, including gastroduodenal ulcers, chronic active gastritis, and even gastric cancer [2]. Its eradication can effectively prevent these diseases [3]. Therefore, effective treatment modalities for H. pylori infection have been continuously explored for a long time.
Currently, a major challenge faced in the pursuit of eradicating H. pylori is increased antibiotic resistance, particularly clarithromycin. Maastricht VI/Florence strongly recommends that a 14-day quadruple therapy should be administered as the first-line therapy for H. pylori infection in countries or regions with clarithromycin resistance exceeding 15% [4]. The recently released Fifth Chinese National Consensus Report on H. pylori infection and management highly recommended bismuth-containing quadruple therapy as the first-line treatment in China [5]. However, despite the improved eradication rate, these quadruple regimens present with several concerns — low compliance due to the long-term use of multiple antibiotics, increased antibiotic resistance, severe adverse effects, and high cost. Therefore, new and effective treatments that reduce adverse events and antibiotic use are urgently warranted [6, 7].
Unlike metronidazole and clarithromycin, which have high drug resistance rates in many areas of China, amoxicillin resistance rate is only 1.2–3.1% [8, 9]. Furthermore, amoxicillin is a pH- and time -dependent antibiotic in that its plasma half-life is short and its bactericidal effect on H. pylori is greatly affected by the gastric pH value [10, 11]. Successive clinical trials in China have reported that compared with bismuth-containing quadruple therapy, high-dose proton pump inhibitor (PPI)–amoxicillin dual therapy has a similar eradication rate and a lower incidence of adverse events [6, 7]. Notably, vonoprazan (VPZ) has stronger gastric acid inhibition than PPI [12], and it is the first potassium-competitive acid blocker used clinically for treating H. pylori infection in Japan in recent years [13]. Studies conducted in Japan found that the H. pylori eradication rates of VPZ and amoxicillin dual regimen (VA-dual) were acceptable and comparable to those of the VPZ, amoxicillin, and clarithromycin triple regimen (VAC-triple) [14]. The H. pylori eradication rate was higher with VA-dual than with VAC-triple when the study was limited to clarithromycin-resistant strains [15]. However, a recent clinical study conducted in Nanchang, China, found that neither 7-day or 10-day VA-dual as the first-line treatment for H. pylori infection achieved satisfactory results [16]. Similarly, another study conducted in Lanzhou, China, found that both 7-day VA-dual and 7-day VAC-triple, did not achieve acceptable eradication rates for H. pylori [17]. Further optimization on the basis of the VA-dual is warranted.
Notably, probiotics have come to be widely used in clinical practice in recent years, and their use for eradicating H. pylori has been proposed as a new treatment regimen. The results of a recent meta-analysis [18] revealed that the combination of standard treatment with the probiotic strain Saccharomyces boulardii (S. boulardii) effectively increased H. pylori eradication rates and reduced adverse events. Therefore, combining S. boulardii supplementation with the VA-dual may improve the eradication rate and reduce treatment-related adverse events.
This prospective study was aimed at evaluating the safety and efficacy aspects of a new combination regimen of VA-dual with S. boulardii (VAS) and comparing the findings with those of bismuth-containing quadruple therapy, which is the standard therapeutic regimen in China. In addition, we also compared the cost-effectiveness of both therapies.
Methods
Statement of ethics and trial registration
This was a prospective, single-center, open-label, randomized controlled study, which was designed according to CONSORT guideline [19]. It was conducted at the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University from September 2021 to April 2022 in accordance with the Declaration of Helsinki. The protocol was approved by the Clinical Medical Technical Ethics Committee of the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University (No. [2021] YLJSC016), and registered in Chinese Clinical trial Registry (No. ChiCTR2100055101 31/12/2021).
Participants and study design
In this study, we included adults aged 18–75 years with confirmed H. pylori infection not having received prior eradication therapy. H. pylori infection was diagnosed on the basis of a positive rapid urease test or a positive 13C-urea breath test (UBT). We excluded patients meeting one or more of the following criteria: (1) previous history of gastrointestinal surgery; (2) previous history of alcohol or substance abuse; (3) severe heart disease, liver failure, or impaired kidney function; (4) allergic to any of the therapeutic drugs; (5) having taken a PPI within 2 weeks or an antibiotic within 4 weeks before the study; and (6) pregnant or breastfeeding women. Before participation in the study, written informed consent was obtained from all patients.
A randomization list was generated using Statistical Package for the Social Sciences version 23.0 (IBM, Armonk, NY, USA), the included patients were randomly assigned to the ECAB group (esomeprazole, clarithromycin, amoxicillin, and bismuth) or the VAS group in 1:1 ratio. Patients in the VAS group were given VPZ (20 mg twice daily; Takeda Pharmaceutical, Tokyo, Japan), amoxicillin (750 mg three times daily; Federal Pharmaceutical, Hong Kong, China), and S. boulardii (250 mg twice daily; Laboratoires BIOCODEX, French) for 10 days. The ECAB group received esomeprazole (20 mg twice daily; AstraZeneca AB, Sweden), clarithromycin (500 mg twice daily; Hengrui Pharmaceutical, Jiangsu, China), amoxicillin (1000 mg twice daily; Federal Pharmaceutical, Hong Kong, China), and bismuth (200 mg twice daily; Anlikon Pharmaceutical, Zhejiang, China) for 14 days.
The medical and demographic data were recorded for all included patients. The occurrence of adverse events was recorded via a questionnaire after the treatment. A UBT was performed for all patients 4–6 weeks after the treatment to assess the H. pylori infection status. H. pylori status was interpreted as positive or negative when the delta was > 4 or < 4 over the baseline, respectively. In addition, if the delta exceeded the baseline range of 4–6, the test was repeated 1 month later.
Sample size calculation
Previous studies have reported an H. pylori eradication rate of 85.0% for VA-dual therapy [14] and 89.7% for ECAB therapy [6]. Considering these eradication rates, we calculated the appropriate sample size for each group as 60 with the non-inferiority margin set to 0.1, α to 0.05, and 1-β to 0.8. Assuming a loss to follow-up rate of 5%, we planned a total sample size of 126 patients with 63 patients in each group.
Study outcomes and statistical analysis
The primary endpoint of this study was the H. pylori eradication rate as elucidated by protocol (PP) and intention-to-treat (ITT) analyses. All randomly assigned patients were included in the ITT analysis. In the ITT analysis, patients who were not followed up with a UBT were considered as having treatment failure. Notably, all patients who had failed follow-up were excluded from PP analysis.
The secondary endpoint comprised the factors associated with eradication rates of the VAS regimen. In the VAS group, eradication rates were compared with binary variables related to sex, alcohol consumption, smoking habit, hypertension, and diabetes. Receiver operating characteristic (ROC) curve analysis was performed for continuous variables [age, weight, height, body surface area (BSA), body mass index (BMI)] to evaluate their relationship with eradication success, and cutoff values of eradication success and the area under the curve (AUC) were calculated. The cutoff values were represented by the threshold at which the sum of sensitivity and specificity minus 1 value was the highest. According to the calculated cutoff value, continuous variables with AUC > 0.5 were divided into classification variables, and the eradication rates above and below the cutoff values were also compared using Pearson’s chi-squared test. BSA was calculated using a modified version of an equation by Mosteller [20]:
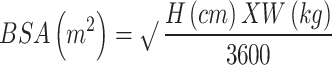 |
For continuous variables, between-group comparisons were performed with t test and the Mann–Whitney test using mean ± standard deviation. For categorical variables, between-group comparisons were done with Fisher’s exact test or Pearson’s chi-square test using numbers with percentages. P values of < 0.05 were considered to indicate statistical significance. All statistical calculations were performed using Power Analysis and Sample Size software version 15.0.5 (NCSS LLC, Kaysville, Utah, USA) and Statistical Package for the Social Sciences version 23.0 (IBM, Armonk, NY, USA).
The third endpoint was the outcome of a cost-effectiveness analysis. Costs are calculated in terms of direct costs (diagnosis, medicines, and the evaluation of eradication). Since all patients in this study were outpatients, and the administration route was oral, the cost of diagnosis and evaluation of eradication were basically the same. Therefore, only medicine cost per patient was defined as the cost, and the first-line eradication rate as per the ITT analysis was defined as the effectiveness [21]. The cost-effectiveness ratio (CER) was used to analyze the cost-effectiveness and is calculated using the following equation:
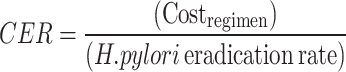 |
For less effective but more affordable treatments, we used the incremental cost-effectiveness ratio (ICER) to evaluate and estimate the additional cost. The ICER was calculated as follows:
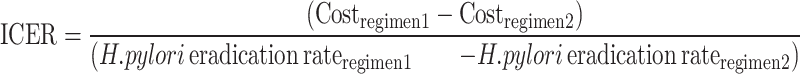 |
All medicine prices were in line with the market prices in April 2022.
Results
Patient enrollment and baseline characteristics
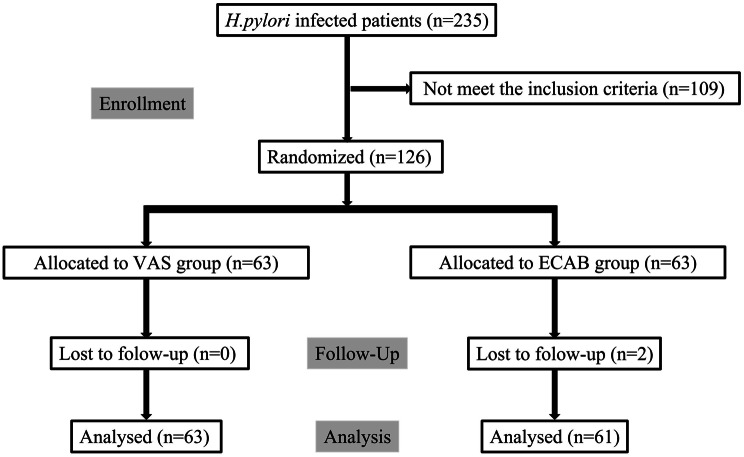
Among the 235 patients with H. pylori infection who visited our outpatient department from September 2021 to April 2022, we excluded 109 patients who did not meet the inclusion criteria, and the remaining 126 patients were randomly assigned to one of the two treatment groups in 1:1 ratio: the VAS group and ECAB group. Among them, 61 patients in the ECAB group and 63 patients in the VAS group successfully completed the regimen. Two patients were lost to follow-up in the former group and did not undergo a UBT for 4 weeks after the treatment (Fig. 1). Notably, patient baseline data for the parameters of height, weight, sex, age, BMI, BSA, smoking habit, alcohol consumption, hypertension and diabetes did not differ between the groups (Table 1).
Fig. 1.
Patient enrollment flow chart
Table 1.
Baseline characteristics of patients in the two treatment groups
| Characteristics | VAS (n = 63) |
ECAB (n = 63) |
P value |
|---|---|---|---|
| Sex (male), n (%) | 36 (57.14) | 39 (61.90) | 0.72 |
| Age (years), mean ± SD | 45.14 ± 11.86 | 44.52 ± 15.10 | 0.80 |
| Height (cm), mean ± SD | 1.65 ± 0.07 | 1.66 ± 0.08 | 0.42 |
| Weight (kg), mean ± SD | 61.64 ± 9.24 | 63.42 ± 10.33 | 0.31 |
| BMI (kg/m2), mean ± SD | 22.72 ± 2.70 | 23.03 ± 2.88 | 0.52 |
| BSA (m2), mean ± SD | 1.675 ± 0.152 | 1.704 ± 0.168 | 0.31 |
| Underlying disease | |||
| Diabetes mellitus, n (%) | 3 (4.76) | 4 (6.35) | 1.00 |
| Hypertension, n (%) | 6 (9.52) | 8 (12.70) | 0.78 |
| Lifestyle, n (%) | |||
| Smoking status, n (%) | 7 (11.11) | 10 (15.87) | 0.60 |
| Alcohol consumption, n (%) | 9 (14.29) | 6 (9.52) | 0.584 |
Abbreviations: SD, standard deviation; BMI, body mass index; BSA, body surface area; VAS, treatment with vonoprazan, amoxicillin, and S. boulardii for 10 days; ECAB, treatment with esomeprazole, clarithromycin, amoxicillin, and bismuth for 14 days
Eradication rates of H. Pylori and adverse events
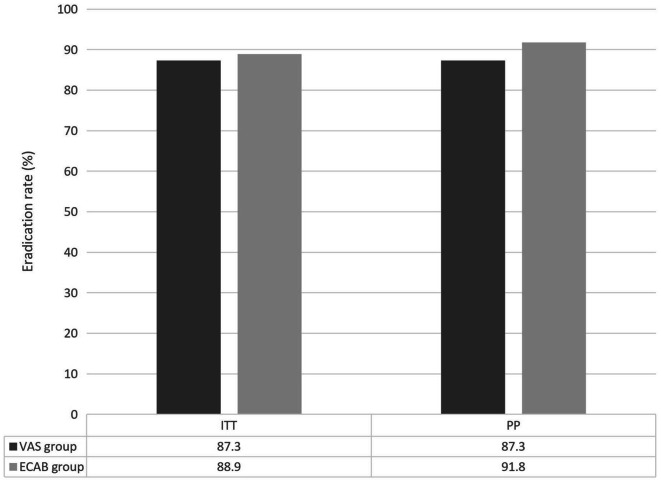
In the VAS group, the eradication rate was 87.3% as per the PP analysis and 87.3% as per the ITT analysis. In the ECAB group, the eradication rate was 91.8% as per the PP analysis and 88.9% as per the ITT analysis (Fig. 2). Notably, ITT (P = 1.00) and PP (P = 0.56) analysis outcomes did not significantly differ between the two groups. Table 2 shows adverse events for all patients. Interestingly, the incidence of adverse events significantly lower in the VAS group (4.8%, 3/63 patients) than in the ECAB group (17.5%, 11/36 patients; P = 0.044). Three patients in the VAS treatment group reported nausea, rash, and abdominal pain (n = one each). Conversely, 11 patients reported adverse events in the ECAB group, including six cases dark stool, three cases of diarrhea, one case of loose stools, and one case of bloating. However, all patients adhered to the prescribed treatment despite these adverse events. All adverse events resolved after the treatment.
Fig. 2.
Eradication rates of VAS and ECAB groups
Table 2.
Adverse events of the two treatment groups
| Adverse events n (%) | VAS (n = 63) | ECAB (n = 63) | P-value |
|---|---|---|---|
| Nausea, n (%) | 1 (1.59) | 0 (0.00) | |
| Bitter taste, n (%) | 0 (0.00) | 1 (1.59) | |
| Skin rash, n (%) | 1 (1.59) | 0 (0.00) | |
| Bloating, n (%) | 0 (0.00) | 1 (1.59) | |
| Diarrhea, n (%) | 0 (0.00) | 3 (4.76) | |
| Abdominal pain, n (%) | 1 (1.59) | 0 (0.00) | |
| Dark stool, n (%) | 0 (0.00) | 6 (9.52) | |
| Total, n (%) | 3 (4.76) | 11 (17.46) | 0.04 |
Abbreviations: VAS, treatment with vonoprazan, amoxicillin, and S. boulardii for 10 days; ECAB, treatment with esomeprazole, clarithromycin, amoxicillin, and bismuth for 14 days
ROC curves and cutoff values for patient factors
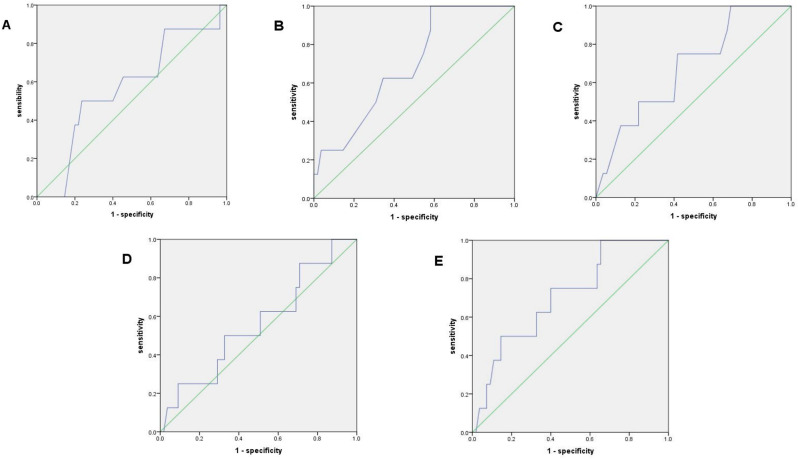
After the ROC analysis of continuous variables for eradication success, the following AUC values were obtained: age, 0.596; height, 0.691; weight, 0.678; BMI, 0.560; and BSA, 0.705; The AUC values of all these aspects were > 0.5 (Fig. 3), and the cutoff values were as follows: age, 53.5 years; height, 160.5 cm; weight, 63.75 kg; BMI, 23.33 kg/m2; and BSA, 1.831 m2.
Fig. 3.
Receiver operating characteristic curves for patient factors and eradication rate of the VAS group. (A) age, (B) height, (C) body weight, (D) body mass index, and (E) body surface area
Relationships between patient factors and eradication success
Table 3 shows the results of the analysis of the relationship between eradication success and all variables. In the VAS group, the eradication rate was significantly higher in (1) shorter patients (height < 1.605) than in taller patients (height ≥ 1.605; 100% vs. 80%; P = 0.023), (2) low-BSA patients (BSA < 1.831) than in high-BSA patients (BSA ≥ 1.831; 94.1% vs. 79.3%; P = 0.037), and non-smokers than in smokers (92.9% vs. 42.9%; P = 0.003). No association was observed between other patient factors and the eradication rate.
Table 3.
The influence of factors on H. pylori eradication with VAS group
| Factors | Eradication success | P-value | |
|---|---|---|---|
| Sex | Male | 91.7% (33/36) | 0.27 |
| Female | 81.5% (22/27) | ||
| Age (years) | < 53.5 | 91.3% (42/46) | 0.20 |
| > 53.5 | 76.5% (13/17) | ||
| Height (cm) | < 1.605 | 100% (23/23) | 0.02 |
| > 1.605 | 80% (32/40) | ||
| Weight (kg) | < 63.575 | 94.1% (32/34) | 0.13 |
| > 63.575 | 79.3% (23/29) | ||
| BMI (kg/m2) | < 23.33 | 90.2% (37/41) | 0.43 |
| > 23.33 | 81.8% (18/22) | ||
| BSA (m2) | < 1.831 | 92.2% (47/51) | 0.04 |
| > 1.831 | 66.7 (8/12) | ||
| Smoking habit | NO | 92.9% (52/56) | 0.00 |
| YES | 42.9% (3/7) | ||
| Alcohol consumption | NO | 90.7% (49/54) | 0.08 |
| YES | 66.7 (6/9) | ||
| Diabetes mellitus | NO | 88.3 (53/60) | 0.34 |
| YES | 66.7 (2/3) | ||
| Hypertension | NO | 86.0% (49/57) | 1.00 |
| YES | 100% (6/6) | ||
Abbreviations: BMI, body mass index; BSA, body surface area; VAS, treatment with vonoprazan, amoxicillin, and S. boulardii for 10 days
Cost-effectiveness analysis
Table 4 shows that the effectiveness of the VAS regimen was non-inferior to the ECAB regimen (87.3% vs. 88.9% P = 1.00). The cost of the VAS therapy was 404.91 Chinese Yuan (CNY) lower than that of the ECAB therapy (404.91 CNY vs. 425.97 CNY). Cost-effectiveness analysis revealed that the CER of the VAS therapy was lesser than that of the ECAB therapy (4.64 vs. 4.79, CNY per percent). In cases wherein the eradication rate of the VAS regimen was not inferior to that of the ECAB regimen and the cost was relatively less, ICER was not calculated.
Table 4.
The cost-effectiveness analysis of VAS and ECAB groups
| VAS group | ECAB group |
|
|---|---|---|
| Cost (direct medical costs per patient) | 404.91 CNY | 425.97 CNY |
| Effectiveness (the first-line eradication rate in the ITT analysis) | 87.3% | 88.9% |
| CER, (CNY per percent) | 4.64 | 4.79 |
Abbreviations: CNY: Chinese Yuan; CER: cost-effectiveness ratio; ITT: intention-to-treat; 10-VAS group, treatment with vonoprazan, amoxicillin, and S. boulardii for 10 days; ECAB group, treatment with esomeprazole, clarithromycin, amoxicillin, and bismuth for 14 days
Discussion
There have been several reports on the efficacy of VA-dual therapy [14–17, 22–25]. As shown in Table 5, only a 7-day retrospective study of the VA-dual (amoxicillin 500 mg 3 times daily) achieved an eradication rate of 92.9% in Japanese adults [22] and a 14-day prospective study of the VA-dual (amoxicillin 1000 mg 2 times daily) achieved an eradication rate of 93.5% in Pakistani adults [24]. Most VA-dual therapies, including amoxicillin (3 g/ day or less) and VPZ (40 mg/ day), did not achieve acceptable eradication rates (> 90%) and were associated with varying degrees of adverse events (10–36.8%), such as nausea, diarrhea, and abdominal pain. Notably, S. boulardii has been extensively studied in many trials in an attempt to further reduce the incidence of adverse events and improve the eradication rate. The most recent meta-analysis [18] revealed that compared with standard eradication regimens, S. boulardii-supplemented regimens were associated with a significantly lower incidence of total adverse events [risk ratio (RR) = 0.47, 95% confidence interval (CI): 0.36–0.61] and higher eradication rate (RR = 1.09; 95% CI: 1.05–1.13).
Table 5.
Reports of dual therapy with Amoxicillin and VPZ in the treatment of H. Pylori infection
| Authors (year) |
Country | Number | Dose of VPZ | Dose of amoxicillin | Duration of treatment |
Eradication rates (ITT) | Adverse events |
|---|---|---|---|---|---|---|---|
| Gotoda T et al. (2020) [14] | Japan | 60 | 20 mg, bid | 750 mg, bid | 7 days | 85.0% |
10% (6/60) |
| Furuta T et al. (2020) [22] | Japan | 56 | 20 mg, bid | 500 mg, tid | 7 days | 92.9% |
16.0% (9/56) |
| Suzuki S et al. (2020) [15] | Japan | 168 | 20 mg, bid | 750 mg, bid | 7 days | 84.5% | 27.4% (46/168) |
| Horii T et al. (2021) [23] | Japan | 19 | 20 mg, bid | 750 mg, bid | 7 days | 84.2% |
36.8% (7/19) |
| Zuberi BF et al. (2022) [24] | Pakistan | 96 | 20 mg, bid | 1000 mg, bid | 14 days | 93.5% |
12.5% (12/96) |
| Chey WD et al. (2022) [25] | US and Europe | 348 | 20 mg, bid | 1000 mg, tid | 14 days | 78.5% | 29.9% (104/348) |
| Hu Y et al. (2022) [16] | China | 37 | 20 mg, bid | 1000 mg, bid | 10 days | 89.2% | 29.7% (11/37) |
| 37 | 20 mg, bid | 1000 mg, tid | 10 days | 81.1% |
24.3% (9/37) |
||
| 24 | 20 mg, bid | 1000 mg, bid | 7 days | 66.7% |
16.7% (4/24) |
||
| 21 | 20 mg, bid | 1000 mg, tid | 7 days | 86.0% |
23.8% (5/21) |
||
| Lin Y et al. (2022) [17] | China | 85 | 20 mg, bid | 750 mg, qid | 7 days | 63.5% |
16.9% (14/85) |
| 84 | 20 mg, bid | 500 mg, qid | 7 days | 58.3% |
13.2% (11/84) |
Abbreviations: ITT, intention-to-treat; VPZ, vonoprazan; bid, twice daily; tid, three times daily; qid, four times daily
To our knowledge, no randomized clinical trials have evaluated the safety and efficacy of VA-dual therapy in combination with S. boulardii to eradicate H. pylori. We conducted the study and found the VAS regimen to be as effective as the ECAB regimen as the first-line treatment for H. pylori infection but with fewer adverse events and lesser cost. The potential mechanisms underlying the eradication of H. pylori by the VAS regimen are as follows: (a) VPZ could achieve a more rapid and sustained acid-inhibitory effect than PPIs [12]. Therefore, when VPZ is used for H. pylori eradication, the ideal pH condition to achieve this can be created quickly and sustained longer in the stomach. Moreover, as VPZ is unaffected by the cytochrome P450 (CYP) 2C19 genotype polymorphism and is mainly metabolized by CYP3A4, it can stably inhibit acid secretion with negligible inter-individual differences [26]. (b) The incidence of primary drug resistance of H. pylori to amoxicillin is low and stable in China (8). In addition, because of the short plasma half-life of amoxicillin, it is administered 3–4 times daily to maintain its minimum inhibitory concentration [27]. (c) S. boulardii can contribute to the eradication of H. pylori by expressing a neuraminidase which reduces surface α(2–3)-linked sialic acid in epithelial cells, thereby preventing H. pylori adhesion in the duodenum epithelial cells [28]. Furthermore, S. boulardii also upregulates the levels of short-chain fatty acids and other antibacterial substances, thus inhibiting the growth and proliferation of H. pylori [29]. (d) Compared with the ECAB regimen, the VAS regimen effectively reduced the treatment time to 10 days and was associated with a reduced drug burden. Furthermore, the VAS regimen was associated with potential advantages in reducing adverse events and improving patient adherence.
Notably, our findings also revealed higher eradication rates in low-BSA patients and non-smokers in the VAS group (> 90%). Body size affects drug metabolism, distribution, and excretion [30]. Pharmacokinetic indicators of some drugs vary for different body sizes and compositions [26]. BSA is a typical indicator of body size and is usually used to calculate drug dose in chemotherapy [20]. Available data support the notion that antimicrobials, such as several β-lactams, are approved for generic administration and should be administered in higher doses to high-BSA patients to better meet their pharmacodynamic goals [30, 31]. The plasma concentration and bioavailability of amoxicillin may be lower in high-BSA patients than in low-BSA patients, thus translating into a lower eradication rate. In addition, previous studies on VPZ-based dual therapy [26] and rabeprazole-based dual therapy [32] have also identified a correlation between lower BSA levels and successful H. pylori eradication, which is consistent with our current results. The optimal dose of the VAS regimen needs further exploration to achieve the best safety and efficacy in high-BSA patients. Moreover, increased pentapeptide secretion in smokers leads to them having a more acidic stomach environment; this increases the proportion of non-replicating bacteria and reduces the efficacy of antibiotics [33, 34]. Second, nicotine increases the production of stomach acids and pepsin and reduces blood flow to the gastric mucosa, thus reducing the dose of antibiotics delivered to the gastric mucosa [35]. Third, smokers are associated with lower drug adherence, which may also increase the likelihood of treatment failure [36]. Therefore, smokers need to quit smoking for better H. pylori eradication.
Furthermore, besides efficacy and safety, treatment cost has become an increasingly important criteria for evaluating a therapeutic regimen. The present study revealed that the VAS regimen was more cost-effective as the first-line treatment for H. pylori eradication than the ECAB regimen, which is currently the first-line treatment for H. pylori infection in China. Its benefits of lower costs and higher eradication rates would effectively reduce the interpretation burden of physicians and the travel costs incurred to patients when coming to healthcare facilities for receiving second-line treatment [21].
This study has some limitations. First, we did not further explore the differences in the H. pylori eradication rate and adverse events between the VA-dual regimen and VAS regimen. Second, we herein explored only the factors listed by us in the study for their impact on the VAS regimen. Other factors potentially related to the eradication rate of the VAS regimen remain unexplored. Third, the optimal dose of amoxicillin and S. boulardii of the VAS regimen and its effect on amoxicillin-resistant strains warrants further study, especially the regimen of VAS [20-mg VPZ twice/day, 1000-mg amoxicillin three times/day, and 250-mg S. boulardii twice/day for 14 days].
In conclusion, addition of S. boulardii to VA-dual for 10 days is as effective as the 14-days bismuth-based quadruple regimen; furthermore, it has fewer adverse events and costs lesser in China. Low-BSA patients and non-smokers are more suitable candidates for this regimen.
Acknowledgements
We thank all our authors listed in this manuscript.
Author contributions
Xiaoyong Wang: conception and design of the study, critical revision, acquisition of data, analysis and interpretation of data, drafting the article, final approval; Jing Yu, Chen Cui: acquisition of data, analysis and interpretation of data, drafting the article, final approval; Kai Ma, Peng Yang, Yizhou Jiang: interpretation of data, revising the article, final approval.
Funding
This work was supported by Clinical Research Project of Changzhou Medical Center of Nanjing Medical University (CMCC202309), Science and Technology Project of Changzhou Health Commission(ZD202336), and The Top Talent of Changzhou “The 14th Five-Year Plan” High-Level Health Talents Training Project (2022CZBJ051).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Clinical Medical Technical Ethics Committee of the Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University (No. [2021] YLJSC016).This study is registered at Chinese Clinical trial Registry. The registration identification number is ChiCTR2100055101.All study participants provided informed written consent prior to study enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Yu and Chen Cui should be considered joint first author.
References
- 1.Man S, Ma Y, Jin C et al. Association between Helicobacter pylori Infection and Diabetes: A Cross-Sectional Study in China. J Diabetes Res. 2020; 2020: 7201379. [DOI] [PMC free article] [PubMed]
- 2.Viazis N, Argyriou K, Kotzampassi K, et al. A four-Probiotics Regimen combined with a standard Helicobacter Pylori-Eradication Treatment reduces Side effects and increases eradication rates. Nutrients. 2022;14:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doorakkers E, Lagergren J, Engstrand L et al. Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a western population. Gut; 67: 2092–6. [DOI] [PubMed]
- 4.Malfertheiner P, Megraud F, Rokkas T et al. European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022:gutjnl–2022.
- 5.Liu WZ, Xie Y, Lu H, et al. Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Zhang Y, Fan L, et al. Eradication efficacy of modified dual therapy compared with Bismuth-Containing Quadruple Therapy as a first-line treatment of Helicobacter pylori. Am J Gastroenterol. 2019;114:437–45. [DOI] [PubMed] [Google Scholar]
- 7.Guan JL, Hu YL, An P, et al. Comparison of high-dose dual therapy with bismuth-containing quadruple therapy in Helicobacter pylori-infected treatment-naive patients: an open-label, multicenter, randomized controlled trial. Pharmacotherapy. 2022;42:224–32. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, Zhu Y, Lu NH. Primary antibiotic resistance of Helicobacter pylori in China. Dig Dis Sci. 2017;62:1146–54. [DOI] [PubMed] [Google Scholar]
- 9.Liu DS, Wang YH, Zhu ZH, et al. Characteristics of Helicobacter pylori antibiotic resistance: data from four different populations. Antimicrob Resist Infect Control. 2019;8:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debets-Ossenkopp YJ, Namavar F, MacLaren DM. Effect of an acidic environment on the susceptibility of Helicobacter pylori to trospectomycin and other antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1995;14:353–5. [DOI] [PubMed] [Google Scholar]
- 11.Midolo PD, Turnidge JD, Munckhof WJ. Is bactericidal activity of Amoxicillin against Helicobacter pylori concentration dependent? Antimicrob Agents Chemother. 1996;40:1327–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects–a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015;42:719–30. [DOI] [PubMed] [Google Scholar]
- 13.Okubo H, Akiyama J, Kobayakawa M, et al. Vonoprazan-based triple therapy is effective for Helicobacter pylori eradication irrespective of clarithromycin susceptibility. J Gastroenterol. 2020;55:1054–61. [DOI] [PubMed] [Google Scholar]
- 14.Gotoda T, Kusano C, Suzuki S, et al. Clinical impact of vonoprazan-based dual therapy with Amoxicillin for H. Pylori infection in a treatment-naïve cohort of junior high school students in Japan. J Gastroenterol. 2020;55:969–76. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose Amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69:1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Xu X, Ouyang YB et al. Optimization of Vonoprazan-Amoxicillin dual therapy for eradicating Helicobacter pyloriinfection in China: a prospective, randomized clinical pilot study. Helicobacter. 2022: e12896. [DOI] [PubMed]
- 17.Lin Y, Xu H, Yun J, et al. The efficacy of vonoprazan combined with different dose Amoxicillin on eradication of Helicobacter pylori: an open, multicenter, randomized clinical study. Ann Transl Med. 2022;10:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou BG, Chen LX, Li B, et al. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: a systematic review and meta-analysis with trial sequential analysis. Helicobacter. 2019;24:e12651. [DOI] [PubMed] [Google Scholar]
- 19.Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. [DOI] [PubMed]
- 20.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 21.Kajihara Y, Shimoyama T, Mizuki I. Analysis of the cost-effectiveness of using Vonoprazan-Amoxicillin-clarithromycin triple therapy for first-line Helicobacter pylori eradication. Scand J Gastroenterol. 2017;52:238–41. [DOI] [PubMed] [Google Scholar]
- 22.Furuta T, Yamade M, Kagami T, et al. Dual therapy with Vonoprazan and Amoxicillin is as effective as Triple Therapy with Vonoprazan, Amoxicillin and Clarithromycin for Eradication of Helicobacter pylori. Digestion. 2020;101:743–51. [DOI] [PubMed] [Google Scholar]
- 23.Horii T, Suzuki S, Takano C, et al. Lower impact of Vonoprazan-Amoxicillin dual therapy on gut microbiota for Helicobacter pylori eradication. J Gastroenterol Hepatol. 2021;36:3314–21. [DOI] [PubMed] [Google Scholar]
- 24.Zuberi BF, Ali FS, Rasheed T, et al. Comparison of Vonoprazan and Amoxicillin Dual Therapy with Standard Triple Therapy with Proton Pump Inhibitor for Helicobacter Pylori eradication: a Randomized Control Trial. Pak J Med Sci. 2022;38:965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chey WD, Mégraud F, Laine L et al. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the US and Europe: Randomized Clinical Trial. Gastroenterology. 2022: S0016-5085(22)00609-6. [DOI] [PubMed]
- 26.Eto H, Suzuki S, Kusano C, et al. Impact of body size on first-line Helicobacter pylori eradication success using Vonoprazan and Amoxicillin dual therapy. Helicobacter. 2021;26:e12788. [DOI] [PubMed] [Google Scholar]
- 27.Furuta T, Sugimoto M, Yamade M, et al. Effect of dosing schemes of Amoxicillin on eradication rates of Helicobacter pylori with Amoxicillin-based triple therapy. J Clin Pharmacol. 2014;54:258–66. [DOI] [PubMed] [Google Scholar]
- 28.Sakarya S, Gunay N. Saccharomyces boulardii expresses neuraminidase activity selective for α2,3-linked sialic acid that decreases Helicobacter pylori adhesion to host cells. APMIS. 2014;122:941–50. [DOI] [PubMed] [Google Scholar]
- 29.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16:2202–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falagas ME, Karageorgopoulos DE. Adjustment of dosing of antimicrobial agents for bodyweight in adults. Lancet. 2010;375:248–51. [DOI] [PubMed] [Google Scholar]
- 31.Alobaid AS, Hites M, Lipman J, et al. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: a structured review. Int J Antimicrob Agents. 2016;47:259–68. [DOI] [PubMed] [Google Scholar]
- 32.Shao QQ, Yu XC, Yu M, et al. Rabeprazole plus Amoxicillin dual therapy is equally effective to bismuth-containing quadruple therapy for Helicobacter pylori eradication in central China: a single-center, prospective, open-label, randomized-controlled trial. Helicobacter. 2022;27:e12876. [DOI] [PubMed] [Google Scholar]
- 33.Parente F, Lazzaroni M, Sangaletti O, et al. Cigarette smoking, gastric acid secretion, and serum pepsinogen I concentrations in duodenal ulcer patients. Gut. 1985;26:1327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina-Infante J, Gisbert JP. Optimizing clarithromycin-containing therapy for Helicobacter pylori in the era of antibiotic resistance. World J Gastroenterol. 2014;20:10338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Endoh K, Leung FW. Effects of smoking and nicotine on the gastric mucosa: a review of clinical and experimental evidence. Gastroenterology. 1994;107:864–78. [DOI] [PubMed] [Google Scholar]
- 36.Shuter J, Bernstein SL. Cigarette smoking is an independent predictor of nonadherence in HIV-infected individuals receiving highly active antiretroviral therapy. Nicotine Tob Res. 2008;10:731–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.