Abstract
Purpose
Elevated red blood cell distribution width (RDW) and decreased hematocrit (HCT) levels are associated with poor prognosis in chronic obstructive pulmonary disease (COPD) patients, but their significance in intensive care unit (ICU) patients with acute exacerbation of COPD (AECOPD) remains uncertain. The RDW/HCT ratio may offer a more comprehensive assessment compared to individual markers, potentially enhancing prognostic accuracy. Furthermore, the utility of RDW/HCT in improving traditional ICU scoring systems remains unexplored.
Patients and Methods
The optimal RDW/HCT ratio cutoff was identified via ROC curve analysis, guiding classification into high and low ratio groups. Univariate and multivariate logistic regression analyses, Kaplan-Meier survival curves, and propensity score matching (PSM) were performed to evaluate the association between RDW/HCT ratio and 28-day all-cause mortality. The predictive value of RDW/HCT ratio compared to traditional ICU scoring systems was assessed using the area under the curve (AUC). Additionally, the eICU database was utilized to validate the robustness of the association between RDW/HCT and mortality in patients with AECOPD.
Results
624 patients were included, with 361 in the low RDW/HCT ratio group and 263 in the high ratio group. PSM yielded 145 matched pairs of patients with balanced baseline characteristics. Multivariate logistic regression analysis revealed that patients with RDW/HCT ratio ≥ 0.473 had significantly higher 28-day all-cause mortality compared to those with RDW/HCT ratio < 0.473 (p < 0.001). Combining RDW/HCT ratio with SOFA score improved the diagnostic accuracy significantly (p=0.029).
Conclusion
The RDW/HCT ratio is an independent predictor of 28-day all-cause mortality in AECOPD patients in the ICU. It can be used for a preliminary assessment before a systematic evaluation of the patient, indicating its potential value in early assessment of disease severity. In a comprehensive evaluation, combining the RDW/HCT ratio with the SOFA score can further enhance predictive accuracy.
Keywords: red blood cell distribution width, hematocrit, chronic obstructive pulmonary disease, intensive care unit, prognosis
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex condition characterized by persistent respiratory symptoms and progressive airflow limitation, primarily driven by lung inflammation.1 Global estimates and literature findings indicate that COPD affects more than 300 million individuals and ranks as the third leading cause of mortality.2 Moreover, with the aging population worldwide, COPD is anticipated to pose an even greater social and economic burden in the coming years.3 Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) denotes a sudden worsening of symptoms in COPD patients, typically marked by heightened breathlessness and increased cough with sputum production, often triggered by pulmonary or systemic inflammatory processes.1 AECOPD is frequently encountered in the intensive care unit (ICU), where it carries a substantial mortality risk ranging from 17.6% to 48.8% among admitted patients.4 Early stratification of patients by severity to implement individualized treatment and care strategies is key to reducing mortality, especially in severe cases of AECOPD.5 Furthermore, recent research indicates that designing specific medical strategies based on different risk stratifications can not only effectively reduce readmission rates post-discharge for AECOPD patients but also significantly improve their health outcomes after discharge.6 Thus, exploring relevant predictive biomarkers for early severity stratification and timely intervention is crucial for improving the prognosis of AECOPD patients in the ICU.
Red blood cell distribution width (RDW) serves as a marker reflecting the variability in the volume of red blood cells. Traditionally, RDW has been utilized to differentiate various types of anemia. Elevated RDW levels typically signify compromised red blood cell production and abnormal red blood cell survival, often linked to metabolic irregularities, oxidative stress, and inflammation.7 Recent investigations have highlighted the prognostic value of elevated RDW in conditions such as systemic lupus erythematosus, viral hepatitis, liver fibrosis, myelodysplastic syndrome, sepsis, and chronic kidney disease.8–12 Moreover, RDW has emerged as an independent predictor of mortality and readmission in AECOPD patients.13,14
Hematocrit (HCT) denotes the volume percentage of blood cells in whole blood, primarily reflecting the total quantity of red blood cells due to the minimal presence of white blood cells and platelets. Studies have associated HCT with adverse outcomes in conditions such as gastric cancer, traumatic brain injury, and sepsis.15–17 Additionally, lower HCT levels have been identified as an independent risk factor for predicting mortality in severe COPD patients.18
However, previous investigations examining the relationship between RDW, HCT, and COPD prognosis may have overlooked critical confounding factors. For instance, patients with peripheral vascular diseases tend to exhibit higher levels of RDW and HCT. Moreover, COPD patients with concurrent peripheral vascular diseases typically experience worse prognoses compared to those without such comorbidities.19–21 Failure to account for these confounding variables may distort the true association between RDW, HCT, and COPD prognosis.
To comprehensively address these confounding factors, we employed propensity score matching (PSM) in our study. PSM is a statistical technique designed to mitigate bias arising from confounding variables by simulating randomization.22 By estimating the probability of each subject being assigned to the intervention group based on their characteristics, generating propensity scores, and then matching subjects between intervention and control groups, PSM effectively balances differences in confounding variables. This enhances comparability between groups, akin to a randomized controlled trial, thus improving research validity and efficiency.
Furthermore, limited research has focused on ICU patients with AECOPD concerning the relationship between RDW, HCT, and COPD prognosis. ICU-COPD patients present significant physiological distinctions compared to non-ICU-COPD patients. Yet, the precise association between RDW, HCT, and AECOPD patients in the ICU remains poorly understood.
Using either RDW or HCT in isolation may be influenced by a range of factors including nutritional status, fluid administration, red blood cell transfusions, and oxidative stress.7,23 However, employing the RDW/HCT ratio can mitigate the impact of individual variables and enhance stability. Moreover, compared to individual parameters, utilizing the RDW/HCT ratio offers insights into both red blood cell characteristics and quantity, rendering the metric more comprehensive and objective. Additionally, employing the ratio of red blood cell property parameters to quantity parameters has demonstrated efficacy in predicting outcomes in conditions such as sepsis, subarachnoid hemorrhage, and various malignancies.24–26 Thus, RDW/HCT holds promise as a predictive indicator.
The Sequential Organ Failure Assessment (SOFA) score, developed by the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine in 1996,27 evaluates the severity and prognosis of patients by assessing the function of six systems: respiratory, cardiovascular, neurological, hepatic, coagulation, and renal. Widely adopted, the SOFA score has become the cornerstone of severity assessment in the ICU. Another commonly used scoring system is SAPS II (Simplified Acute Physiology Score II),28 comprising 17 variables related to the patient’s physiological status and clinical information. Despite their extensive use, variations in the performance of SOFA and SAPS II persist across different disease populations.29 Enhancing the accuracy of these scoring systems and exploring their applicability in diverse disease contexts remain areas of ongoing investigation.30
This study endeavors to elucidate the relationship between RDW/HCT and the 28-day all-cause mortality rate among AECOPD patients in the ICU, aiming to furnish a straightforward and efficacious predictive marker for the early identification of critically ill AECOPD patients in the ICU. Furthermore, we sought to compare the efficacy of RDW/HCT with that of SOFA and SAPS II, as well as examine the potential benefits of their combined application.
Materials and Methods
Data Source
This study utilized data from two sources, the MIMIC-IV (v2.2) and eICU (v2.0) databases. Analysis and modeling were primarily conducted using the MIMIC-IV database, with the eICU database used for validation purposes. MIMIC-IV is a large, single-center medical database maintained by the Laboratory for Computational Physiology at the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center, which is affiliated with Harvard Medical School.31 The eICU database contains a large multicenter dataset with records from over 200,000 ICU admissions from 2014 to 2015.32 All information in the databases has been anonymized to ensure patient privacy, following protocols that included obtaining written informed consent from participants. Zhiwei Long, authorized to access both databases (name ID: 12635073, record ID: 58429392), has completed all necessary training. The retrospective design of this study and the thorough anonymization of all patient information led the Ethics Review Board at the First Affiliated Hospital of Guangzhou Medical University to waive the need for ethical review.
Inclusion and Exclusion Criteria
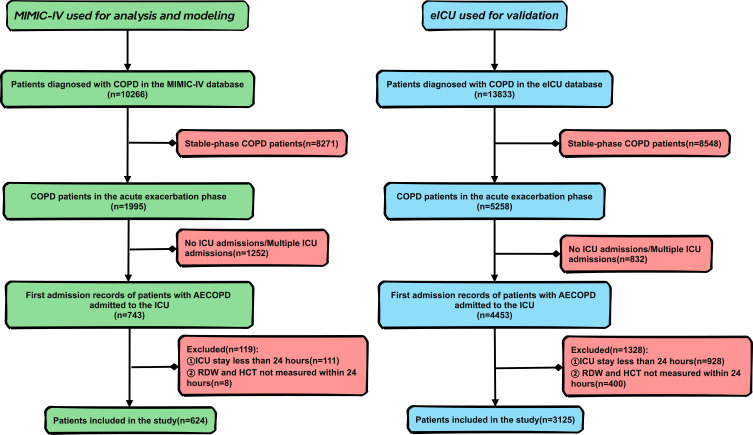
Inclusion criteria encompassed patients diagnosed with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) based on the International Classification of Disease, Tenth Revision. Exclusion criteria included patients not admitted to the ICU, ICU stays less than 24 hours, RDW/HCT measurements not taken within 24 hours of ICU admission, and instances of more than 15% missing data. Additionally, for patients with multiple ICU admissions, only the first hospitalization record was selected. After screening, the MIMIC-IV database included 624 patients, while the eICU database included 3125 patients. Details of the inclusion process can be seen in Figure 1.
Figure 1.
Inclusion process of study population.
Data Extraction
Structured Query Language (SQL 16.0) was employed to extract information on patients admitted to the ICU within 24 hours, encompassing demographics (age, gender, height, weight, ethnicity), vital signs (heart rate, respiratory rate, blood pressure, oxygen saturation), laboratory indicators (RDW, HCT, WBC, PLT, electrolytes, renal function, blood gas analysis, liver function tests), comorbidities, prescriptions, mechanical ventilation status, and scoring systems (SOFA, SAPS II).
Primary and Secondary Research Objectives
The primary objective of this study is to evaluate the relationship between RHR and the 28-day mortality rate among patients with AECOPD in ICU. The secondary objectives are to compare the performance of RHR with classic scoring systems (SOFA and SAPS II) and to explore their combined application in the ICU setting.
Statistical Analysis
Normality of continuous variables was assessed using the Kolmogorov–Smirnov test. Continuous variables are presented as median (interquartile range), while categorical variables are presented as percentages. Univariate analysis involved comparison of continuous variables using the t-test or Mann–Whitney test, and categorical variables using the chi-square or Fisher’s exact test. Receiver operating characteristic (ROC) curves were generated, and the Youden index was calculated to determine the optimal cutoff value for the RDW/HCT ratio (RHR), dividing patients into high and low RHR groups. The predictive abilities of RHR, SOFA score, and SAPS II score were evaluated by calculating the area under the curve (AUC).
Univariate and multivariate logistic regression analyses were conducted to assess the association between RHR and 28-day mortality. Variance inflation factor (VIF) was used to test for multicollinearity, with VIF < 5 indicating model stability. Confounding factors were selected based on specific criteria. Five models were constructed, progressively adjusting for relevant covariates.
To balance potential confounding factors between the high and low RHR groups, a 1:1 propensity score matching (PSM) was conducted. Logistic regression was used to calculate propensity scores for variables such as gender, age, ethnicity, and physiological parameters. Ultimately, 145 pairs of patients were successfully matched in the MIMIC-IV database. The reliability of PSM was assessed using standardized mean differences (SMD), density plots, and histograms based on propensity scores.
Kaplan-Meier (KM) survival curves were employed to compare 28-day mortality between the high and low RHR groups before and after PSM, with differences assessed using the Log rank test. Subgroup analysis was conducted based on relevant clinical variables. To ensure the reliability of the results, logistic regression was used to evaluate the performance of RHR in the eICU database.
Statistical analyses were performed using SPSS version 25.0, MedCalc statistical software version 20.022, Storm statistical platform, R version 4.3.0, and GraphPad Prism version 9.5.1. A two-tailed test was conducted, and P < 0.05 was considered statistically significant.
Results
Original Cohort
Figure 1 illustrates the inclusion process for the study population in the MIMIC-IV database, which encompasses a total of 624 patients. Table 1 provides a summary of the baseline characteristics of both survivors and non-survivors. Among the 624 patients, the median age was 72.2 years, with 48.7% being male and 51.3% female. The majority were of White ethnicity (67.6%), followed by Black (11.4%) and other ethnicities (21.0%). Of these, 499 were survivors, while 125 were non-survivors. Non-survivors tended to be older, had higher heart rates, wider red blood cell distribution width, lower hematocrit, significantly elevated white blood cell counts, reduced platelet counts, higher levels of creatinine and anion gap. Moreover, non-survivors presented with more cases of pulmonary embolism and sepsis, and received non-invasive ventilation more frequently. Furthermore, the non-survivor group had higher SOFA and SAPS II scores and longer ICU stays. All these differences were statistically significant with p < 0.05.
Table 1.
Baseline Data of the Original Cohort
| Variable | Total (n=624) | Survivors (n=499) | Non-Survivors (n=125) | P-value |
|---|---|---|---|---|
| Demographic | ||||
| Age | 72.2 (65.6, 80.5) | 71.2 (64.3, 78.3) | 76.3 (68.7, 85.1) | <0.001 |
| Male | 304 (48.7) | 249 (49.9) | 55 (44.0) | 0.238 |
| Ethnicity | ||||
| White | 422 (67.6) | 338 (67.8) | 84 (67.2) | 0.670 |
| Black | 71 (11.4) | 59 (11.8) | 12 (9.6) | |
| Others | 131 (21.0) | 102 (20.4) | 29 (23.2) | |
| Vital signs | ||||
| HR (beats/min) | 107.0 (93.0, 122.0) | 106.0 (92.0, 121.0) | 112.0 (95.0, 130.0) | 0.025 |
| RR (beats/min) | 30.0 (26.0, 34.0) | 30.0 (26.0, 34.0) | 31.0 (27.0, 35.0) | 0.116 |
| SBP (mmHg) | 147.0 (132.0, 164.0) | 147.0 (132.5, 165.0) | 147.0 (132.0, 159.0) | 0.497 |
| DBP (mmHg) | 93.0 (80.0, 107.0) | 93.0 (80.0, 107.0) | 92.0 (81.0, 104.6) | 0.372 |
| MAP (mmHg) | 104.0 (93.0, 118.0) | 104.0 (93.0, 119.0) | 103.0 (92.0, 117.8) | 0.293 |
| SpO2 (%) | 100.0 (99.0, 100.0) | 100.00 (98.0, 100.0) | 100.0 (99.0, 100.0) | 0.181 |
| Laboratory | ||||
| RDW (%) | 15.3 (14.0, 17.0) | 15.1 (13.9, 16.9) | 15.8 (14.5, 17.5) | 0.001 |
| HCT (%) | 34.5 (29.8, 39.7) | 35.2 (30.2, 40.2) | 32.1 (27.6, 36.7) | <0.001 |
| RDW/HCT | 0.45 (0.37, 0.56) | 0.44 (0.36, 0.53) | 0.49 (0.41, 0.60) | <0.001 |
| WBC (K/uL) | 11.9 (8.4, 16.1) | 11.4 (8.3, 15.7) | 13.0 (9.0, 17.5) | 0.038 |
| PLT (K/uL) | 206.0 (153.0, 266.0) | 209.0 (157.5, 268.0) | 185.0 (137.0, 245.0) | 0.010 |
| Potassium (mEq/L) | 4.5 (4.2, 5.0) | 4.5 (4.1, 5.0) | 4.5 (4.2, 5.1) | 0.460 |
| Sodium (mEq/L) | 140.0 (137.0, 143.0) | 140.0 (137.0, 143.0) | 141.0 (138.0, 144.0) | 0.162 |
| Glucose (mg/dL) | 158.50 (128.0, 205.0) | 160.0 (129.0, 205.0) | 153.0 (126.0, 201.0) | 0.458 |
| Cr (mg/dL) | 1.1 (0.8, 1.6) | 1.1 (0.8, 1.5) | 1.3 (0.8, 2.1) | 0.005 |
| PH | 7.39 (7.35, 7.44) | 7.39 (7.35, 7.44) | 7.39 (7.34, 7.43) | 0.401 |
| PaCO2 (mmHg) | 55.0 (46.0, 69.1) | 55.0 (46.0, 68.0) | 57.0 (47.0, 72.0) | 0.225 |
| Anion gap (mEq/L) | 15.0 (13.0, 18.0) | 15.0 (13.0, 18.0) | 16.0 (13.0, 19.0) | 0.046 |
| Bicarbonate (mEq/L) | 27.0 (23.0, 31.0) | 27.0 (23.0, 31.0) | 26.0 (23.0, 30.0) | 0.650 |
| Comorbidities | ||||
| Hypertension | 192 (30.8) | 155 (31.1) | 37 (29.6) | 0.751 |
| Diabetes | 62 (9.9) | 55 (11.0) | 7 (5.6) | 0.070 |
| Asthma | 30 (4.8) | 27 (5.4) | 3 (2.4) | 0.159 |
| Pulmonary embolism | 24 (3.9) | 13 (2.6) | 11 (8.8) | 0.003 |
| Anemia | 99 (15.9) | 79 (15.8) | 20 (16.0) | 0.963 |
| Hematological malignancies | 18 (2.9) | 12 (2.4) | 6 (4.8) | 0.258 |
| Connective tissue diseases | 23 (3.7) | 20 (4.0) | 3 (2.4) | 0.557 |
| Peripheral vascular diseases | 45 (7.2) | 37 (7.4) | 8 (6.4) | 0.695 |
| Myocardial infarction | 75 (12.0) | 54 (10.8) | 21 (16.8) | 0.066 |
| Liver disease | 18 (2.9) | 14 (2.8) | 4 (3.2) | 1.000 |
| Chronic kidney disease | 60 (9.6) | 45 (9.0) | 15 (12.0) | 0.312 |
| Malignant tumors | 46 (7.4) | 37 (7.4) | 9 (7.2) | 0.934 |
| Sepsis | 139 (22.3) | 96 (19.2) | 43 (34.4) | <0.001 |
| Prescriptions* | ||||
| Corticosteroid | 10 (1.6) | 10 (2.0) | 0 (0.0) | 0.231 |
| Bronchodilators | 410 (65.7) | 334 (66.9) | 76 (60.8) | 0.196 |
| Vasopressors | 20 (3.2) | 13 (2.6) | 7 (5.6) | 0.157 |
| Intravenous fluids | 438 (70.2) | 342 (68.5) | 96 (76.8) | 0.071 |
| Blood transfusions | 48 (7.7) | 35 (7.0) | 13 (10.4) | 0.204 |
| Mechanical ventilation | ||||
| Invasive | 216 (34.6) | 174 (34.9) | 42 (33.6) | 0.790 |
| Non-invasive | 147 (23.6) | 108 (21.6) | 39 (31.2) | 0.024 |
| Score | ||||
| SOFA | 4.0 (2.0, 7.0) | 4.0 (2.0, 7.0) | 6.0 (3.0, 9.0) | <0.001 |
| SAPSII | 37.0 (29.0, 45.0) | 35.0 (28.0, 43.0) | 43.0 (37.0, 52.0) | <0.001 |
| ICU stay (day) | 3.0 (1.8, 6.0) | 2.7 (1.7, 5.5) | 4.1 (2.7, 8.6) | <0.001 |
Notes: *All prescriptions were issued within 24 hours of ICU admission for patients. A p-value less than 0.05 is considered statistically significant.
Abbreviations: HR, Heart Rate; RR, Respiratory Rate; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; MAP, Mean Arterial Pressure; RDW, Red Blood Cell Distribution Width; HCT, Hematocrit; WBC, White Blood Cell; PLT, Platelet; Cr, Creatinine; SOFA, Sequential Organ Failure Assessment; SAPSII, Simplified Acute Physiology ScoreII.
Before Propensity Score Matching
ROC curve analysis was conducted to assess the correlation between RHR and 28-day mortality, with detailed results provided in Table S1 and Figure S1. The calculated AUC area was 0.624 (95% CI, 0.585–0.662), with a maximum Youden index of 0.213. Sensitivity was determined to be 62.12%, specificity was 59.20%, and the optimal cutoff value was identified as 0.473. Using this cutoff value, the study population was stratified into a low RHR group (RHR < 0.473) consisting of 361 individuals and a high RHR group (RHR ≥ 0.473) consisting of 263 individuals. Their baseline characteristics are summarized in Table 2. The high RHR group exhibited a significantly higher proportion of males, lower diastolic and mean arterial pressures, and higher oxygen saturation. Laboratory tests revealed significantly elevated creatinine levels and decreased PaCO2 levels in the high RHR group. Additionally, the high RHR group showed significantly higher rates of hypertension, hematological malignancies, peripheral vascular diseases, and chronic kidney disease. Moreover, a higher proportion of patients in the high RHR group received red blood cell transfusions within 24 hours. Furthermore, the high RHR group demonstrated higher SOFA and SAPS II scores, as well as a greater 28-day mortality rate. All these differences were statistically significant with p < 0.05.
Table 2.
The Baseline Data for the High RHR Group and Low RHR Group Before PSM
| Variable | RHR<0.473 (n=361) | RHR≥0.473(n=263) | P-value |
|---|---|---|---|
| Demographic | |||
| Age | 71.7 (65.5, 78.6) | 73.8 (65.9, 82.1) | 0.139 |
| Male | 304 (48.7) | 191 (52.9) | 0.014 |
| Ethnicity | |||
| White | 237 (65.7) | 185 (70.4) | 0.416 |
| Black | 42 (11.6) | 29 (11.0) | |
| Others | 82 (22.7) | 49 (18.6) | |
| Vital signs | |||
| HR (beats/min) | 107.0 (93.0, 123.0) | 106.0 (90.0, 121.0) | 0.343 |
| RR (beats/min) | 30.0 (27.0, 35.0) | 30.0 (26.0, 33.0) | 0.300 |
| SBP (mmHg) | 148.0 (134.0, 164.0) | 145.0 (130.5, 161.5) | 0.187 |
| DBP (mmHg) | 95.0 (81.0, 107.0) | 89.0 (78.0, 104.6) | 0.011 |
| MAP (mmHg) | 105.0 (95.0, 120.0) | 102.0 (91.0, 117.8) | 0.012 |
| SpO2 (%) | 99.0 (98.0, 100.0) | 100.0 (99.0, 100.0) | <0.001 |
| Laboratory | |||
| WBC (K/uL) | 12.1 (8.7, 16.4) | 11.2 (7.9, 15.8) | 0.93 |
| PLT (K/uL) | 208.0 (166.0, 262.0) | 198.0 (131.5, 273.0) | 0.101 |
| Potassium (mEq/L) | 4.6 (4.2, 5.0) | 4.5 (4.1, 5.1) | 0.359 |
| Sodium (mEq/L) | 140.0 (137.0, 143.0) | 140.0 (137.0, 143.0) | 0.584 |
| Glucose (mg/dL) | 159.0 (130.0, 209.0) | 157.0 (125.5, 197.5) | 0.262 |
| Cr (mg/dL) | 1.0 (0.7, 1.4) | 1.3 (0.9, 2.2) | <0.001 |
| PH | 7.39 (7.35, 7.43) | 7.40 (7.35, 7.45) | 0.062 |
| PaCO2 (mmHg) | 56.0 (46.0, 71.0) | 54.0 (45.5, 66.0) | 0.036 |
| Anion gap (mEq/L) | 15.0 (13.0, 18.0) | 16.0 (13.0, 18.0) | 0.254 |
| Bicarbonate (mEq/L) | 27.0 (23.0, 31.0) | 27.0 (23.0, 30.0) | 0.740 |
| Comorbidities | |||
| Hypertension | 132 (36.6) | 60 (22.81) | <0.001 |
| Diabetes | 42 (11.6) | 20 (7.60) | 0.097 |
| Asthma | 14 (3.9) | 16 (6.08) | 0.203 |
| Pulmonary embolism | 14 (3.9) | 10 (3.80) | 0.961 |
| Anemia | 59 (16.3) | 40 (15.21) | 0.702 |
| Hematological malignancies | 2 (0.6) | 16 (6.1) | <0.001 |
| Connective tissue diseases | 13 (3.6) | 10 (3.8) | 0.895 |
| Peripheral vascular diseases | 17 (4.7) | 28 (10.7) | 0.005 |
| Myocardial infarction | 41 (11.4) | 34 (12.9) | 0.551 |
| Liver disease | 8 (2.2) | 10 (3.8) | 0.242 |
| Chronic kidney disease | 26 (7.2) | 34 (12.9) | 0.017 |
| Malignant tumors | 25 (6.9) | 21 (8.0) | 0.617 |
| Sepsis | 75 (20.8) | 64 (24.3) | 0.291 |
| Prescriptions* | |||
| Corticosteroid | 4 (1.1) | 6 (2.3) | 0.249 |
| Bronchodilators | 246 (68.1) | 164 (62.4) | 0.133 |
| Vasopressors | 12 (3.3) | 8 (3.0) | 0.843 |
| Intravenous fluids | 244 (67.6) | 194 (73.8) | 0.096 |
| Blood transfusions | 5 (1.4) | 43 (16.4) | <0.001 |
| Mechanical ventilation | |||
| Invasive | 130 (36.0) | 86 (32.7) | 0.391 |
| Non-invasive | 92 (25.5) | 55 (20.9) | 0.184 |
| Score | |||
| SOFA | 4.0 (2.0, 6.0) | 5.0 (3.0, 8.0) | <0.001 |
| SAPSII | 36.0 (28.0, 43.0) | 39.0 (31.0, 47.5) | 0.001 |
| ICU stay (day) | 2.9 (1.7, 6.0) | 3.2 (1.8, 6.1) | 0.321 |
| 28-day mortality (%) | 51 (14.1) | 74 (28.1) | <0.001 |
Notes: *All prescriptions were issued within 24 hours of ICU admission for patients. A p-value less than 0.05 is considered statistically significant.
Abbreviations: HR, Heart Rate; RR, Respiratory Rate; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; MAP, Mean Arterial Pressure; WBC, White Blood Cell; PLT, Platelet; Cr, Creatinine; SOFA, Sequential Organ Failure Assessment; SAPSII, Simplified Acute Physiology ScoreII.
After Propensity Score Matching
To mitigate potential confounding factors, a 1:1 nearest neighbor propensity score matching (PSM) was conducted on the high and low RHR groups. Ultimately, 145 pairs of patients were obtained, and their characteristics are presented in Table 3. Following PSM, all baseline characteristics between the two groups were balanced (P > 0.05). Standardized mean differences (SMD) before and after matching are provided in Figure S2, indicating that most variables had an SMD < 0.1 between the high and low RHR groups after matching, signifying effective matching results. Propensity score probability density distribution plots and histograms before and after matching displayed similar shapes and heights for both groups, further confirming the quality of matching. These density plots and histograms are also provided in Figure S3 and S4.
Table 3.
The Baseline Data for the High RHR Group and Low RHR Group After PSM
| Variable | RHR<0.473(n=145) | RHR≥0.473(n=145) | P-value |
|---|---|---|---|
| Demographic | |||
| Age | 72.1 (66.1, 80.6) | 74.8 (63.8, 83.5) | 0.513 |
| Male | 58 (40.0) | 64 (44.1) | 0.475 |
| Ethnicity | |||
| White | 104 (71.7) | 102 (70.3) | 0.888 |
| Black | 19 (13.1) | 18 (12.4) | |
| Others | 22 (15.2) | 25 (17.2) | |
| Vital signs | |||
| HR (beats/min) | 106.0 (93.0, 125.0) | 108.0 (91.0, 121.0) | 0.950 |
| RR (beats/min) | 30.0 (27.0, 35.0) | 30.0 (26.0, 35.0) | 0.576 |
| SBP (mmHg) | 148.0 (131.0, 163.0) | 148.0 (133.0, 164.0) | 0.861 |
| DBP (mmHg) | 94.0 (81.0, 107.0) | 90.0 (79.0, 105.0) | 0.224 |
| MAP (mmHg) | 103.0 (94.0, 122.0) | 103.0 (92.0, 117.8) | 0.249 |
| SpO2 (%) | 100.0 (99.0, 100.0) | 100.0 (99.0, 100.0) | 0.904 |
| Laboratory | |||
| WBC (K/uL) | 11.9 (8.7, 15.3) | 10.9 (7.9, 15.6) | 0.500 |
| PLT (K/uL) | 219.0 (174.0, 264.0) | 213.0 (151.0, 271.0) | 0.302 |
| Potassium (mEq/L) | 4.5 (4.2, 5.1) | 4.5 (4.1, 5.0) | 0.236 |
| Sodium (mEq/L) | 141.0 (138.0, 143.0) | 140.0 (137.0, 144.0) | 0.571 |
| Glucose (mg/dL) | 150.0 (126.0, 197.0) | 152.0 (122.0, 191.0) | 0.688 |
| Cr (mg/dL) | 1.1 (0.8, 1.5) | 1.1 (0.8, 1.8) | 0.457 |
| PH | 7.40 (7.35, 7.45) | 7.39 (7.35, 7.44) | 0.453 |
| PaCO2 (mmHg) | 56.0 (46.0, 71.0) | 56.0 (46.0, 68.0) | 0.890 |
| Anion gap (mEq/L) | 15.0 (13.0, 18.0) | 15.0 (13.0, 18.0) | 0.561 |
| Bicarbonate (mEq/L) | 27.0 (23.0, 32.0) | 27.0 (23.0, 30.0) | 0.609 |
| Comorbidities | |||
| Hypertension | 40 (27.6) | 37 (25.5) | 0.690 |
| Diabetes | 18 (12.4) | 19 (13.1) | 0.860 |
| Asthma | 8 (5.5) | 8 (5.5) | 1.000 |
| Pulmonary embolism | 6 (4.1) | 4 (2.8) | 0.520 |
| Anemia | 28 (19.3) | 23 (15.9) | 0.441 |
| Hematological malignancies | 1 (0.7) | 3 (2.1) | 0.615 |
| Connective tissue diseases | 5 (3.5) | 5 (3.5) | 1.000 |
| Peripheral vascular diseases | 11 (7.6) | 9 (6.2) | 0.643 |
| Myocardial infarction | 18 (12.4) | 19 (13.1) | 0.860 |
| Liver disease | 2 (1.4) | 3 (2.1) | 1.000 |
| Chronic kidney disease | 15 (10.3) | 14 (9.7) | 0.845 |
| Malignant tumors | 10 (6.9) | 10 (6.9) | 1.000 |
| Sepsis | 29 (20.0) | 34 (23.5) | 0.476 |
| Prescriptions* | |||
| Corticosteroid | 3 (2.1) | 4 (2.8) | 1.000 |
| Bronchodilators | 90 (62.1) | 98 (67.6) | 0.325 |
| Vasopressors | 3 (2.1) | 4 (2.8) | 1.000 |
| Intravenous fluids | 97 (66.9) | 103 (71.0) | 0.446 |
| Blood transfusions | 5 (3.5) | 4 (2.8) | 1.000 |
| Mechanical ventilation | |||
| Invasive | 42 (29.0) | 46 (31.7) | 0.609 |
| Non-invasive | 39 (26.9) | 36 (24.8) | 0.687 |
| Score | |||
| SOFA | 4.0 (2.0, 6.0) | 4.0 (3.0, 7.0) | 0.321 |
| SAPSII | 36.0 (28.0, 44.0) | 36.0 (28.0, 46.0) | 0.643 |
| ICU stay (day) | 3.0 (1.7, 6.2) | 3.2 (1.9, 6.0) | 0.688 |
| 28-day mortality (%) | 20 (13.8) | 43 (29.7) | 0.001 |
Notes: *All prescriptions were issued within 24 hours of ICU admission for patients. A p-value less than 0.05 is considered statistically significant.
Abbreviations: HR, Heart Rate; RR, Respiratory Rate; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; MAP, Mean Arterial Pressure; WBC, White Blood Cell; PLT, Platelet; Cr, Creatinine; SOFA, Sequential Organ Failure Assessment; SAPSII, Simplified Acute Physiology ScoreII.
Relationship Between RHR and 28-Days Mortality
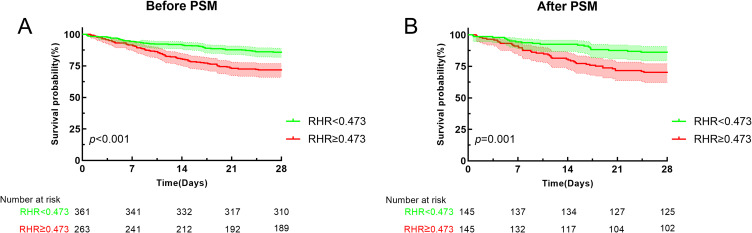
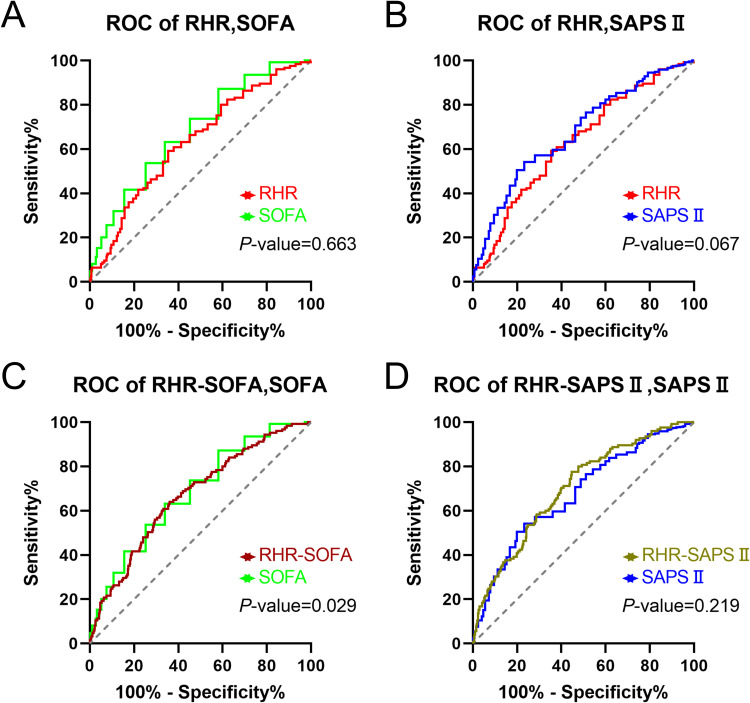
The association between RHR and 28-day mortality was assessed through logistic regression, with the variance inflation factor (VIF) recorded in Table S2. VIF < 5 was considered acceptable Table 4 illustrates the relationship between RHR as a continuous variable and 28-day mortality before and after PSM. Results indicated a positive correlation between RHR and 28-day mortality both before and after PSM. Before PSM (Model 1: OR=12.603, 95% CI: 3.679–43.172, p<0.001; Model 2: OR=15.648, 95% CI: 4.326–56.604, p<0.001; Model 3: OR=9.934, 95% CI: 2.591–38.080, p=0.001; Model 4: OR=17.830, 95% CI: 4.359–72.927, p<0.001; Model 5: OR=11.152, 95% CI: 2.386–52.130, p=0.002). After PSM (Model 1: OR=52.959, 95% CI: 6.690–419.222, p<0.001; Model 2: OR=67.585, 95% CI: 7.646–597.413, p<0.001; Model 3: OR=65.992, 95% CI: 6.427–677.622, p<0.001; Model 4: OR=37.717, 95% CI: 3.584–369.107, p=0.002; Model 5: OR=61.515, 95% CI: 5.034–751.686, p=0.001). Table 5 presents the results when RHR was treated as a categorical variable, revealing that the high RHR group (RHR ≥ 0.473) exhibited significantly higher 28-day mortality rates compared to the low RHR group (RHR < 0.473) both before and after PSM. Before PSM (Model 1: OR=2.380, 95% CI: 1.595–3.550, p<0.001; Model 2: OR=2.296, 95% CI: 1.524–3.461, p<0.001; Model 3: OR=2.367, 95% CI: 1.544–3.629, p<0.001; Model 4: OR=2.651, 95% CI: 1.699–4.136, p<0.001; Model 5: OR=2.458, 95% CI: 1.531–3.945, p<0.001). After PSM (Model 1: OR=2.635, 95% CI: 1.458–4.760, p=0.001; Model 2: OR=2.608, 95% CI: 1.419–4.791, p=0.002; Model 3: OR=2.813, 95% CI: 1.506–5.255, p=0.001; Model 4: OR=2.697, 95% CI: 1.423–5.110, p=0.002; Model 5: OR=2.652, 95% CI: 1.462–5.647, p=0.003). Figure 2 illustrates the Kaplan-Meier survival curves for the high and low RHR groups before and after PSM. Before PSM, it is visually apparent that the high RHR group exhibited a higher 28-day mortality rate, with the Log rank test yielding a p-value < 0.001. After PSM, the high RHR group continued to display a higher 28-day mortality rate (p=0.001). Table S3 and Figure S5 provide details on the ROC curves for RHR, RDW, and HCT. The findings indicated that the AUC area for RHR was higher compared to RDW (0.624 vs 0.593, p=0.098) and HCT (0.624 vs 0.616, p=0.537), albeit not significantly different. Additionally, the predictive capabilities of RHR, SOFA score, and SAPS II score for 28-day mortality were compared. As depicted in Figure 3 and summarized in Table 6, the AUC areas for SOFA score, SAPS II score, and RHR were 0.688, 0.640, and 0.624, respectively. No significant differences in AUC areas were observed between RHR and SOFA score or SAPS II score (p > 0.05). When RHR was combined with SOFA and SAPS II scores, the AUC areas were 0.670 and 0.701, respectively. Notably, the combination of RHR and SOFA score significantly enhanced the AUC area compared to SOFA score alone (p=0.029).
Table 4.
Multivariate Logistic Regression Analysis of the 28-Day All-Cause Mortality Rate in ICU Patients with AECOPD(RHR as a Continuous Variable)
| Model 1 | OR | Model 2 | OR | Model 3 | OR | Mode 4 | OR | Model 5 | OR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Before PSM | <0.001 | 12.603(3.679~43.172) | <0.001 | 15.648(4.326~56.604) | 0.001 | 9.934(2.591~38.080) | <0.001 | 17.830(4.359~72.927) | 0.002 | 11.152(2.386~52.130) |
| PSM | <0.001 | 52.959(6.690~419.222) | <0.001 | 67.585(7.646~597.413) | <0.001 | 65.992(6.427~677.622) | 0.002 | 37.717(3.584~369.107) | 0.001 | 61.515(5.034~751.686) |
Notes: Model 1 was not adjusted for any variables. Model 2 was adjusted for gender, age, and ethnicity. Model 3 was adjusted for hypertension, diabetes, asthma, pulmonary embolism, anemia, hematologic malignancy, connective tissue disease, myocardial infarction, peripheral vascular disease, liver disease, chronic kidney disease, malignancy, and sepsis. Model 4 was adjusted for heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, SpO2, white blood cell, platelet, potassium, sodium, glucose, creatinine, pH, PaCO2, anion gap, and bicarbonate. Model 5 was adjusted for age, gender, ethnicity, white blood cell, platelet, creatinine, hypertension, anemia, hematologic malignancy, connective tissue disease, peripheral vascular disease, myocardial infarction, liver disease, chronic kidney disease, sepsis, vasopressors, corticosteroids, intravenous fluids, red blood cell transfusions, SOFA, and SAPSII.
Table 5.
Multivariate Logistic Regression Analysis of the 28-Day All-Cause Mortality Rate in ICU Patients with AECOPD(RHR as a Categorical Variable)
| Model 1 | OR | Model 2 | OR | Model 3 | OR | Model 4 | OR | Model 5 | OR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Before PSM | <0.001 | 2.380(1.595~3.550) | <0.001 | 2.296(1.524~3.461) | <0.001 | 2.367(1.544~3.629) | <0.001 | 2.651(1.699~4.136) | <0.001 | 2.458(1.531~3.945) |
| PSM | 0.001 | 2.635(1.458~4.760) | 0.002 | 2.608(1.419~4.791) | 0.001 | 2.813(1.506~5.255) | 0.002 | 2.697(1.423~5.110) | 0.003 | 2.652(1.462~5.647) |
Notes: Model 1 was not adjusted for any variables. Model 2 was adjusted for gender, age, and ethnicity. Model 3 was adjusted for hypertension, diabetes, asthma, pulmonary embolism, anemia, hematologic malignancy, connective tissue disease, myocardial infarction, peripheral vascular disease, liver disease, chronic kidney disease, malignancy, and sepsis. Model 4 was adjusted for heart rate, respiratory rate, systolic blood pressure, diastolic blood pressure, SpO2, white blood cell, platelet, potassium, sodium, glucose, creatinine, pH, PaCO2, anion gap, and bicarbonate. Model 5 was adjusted for age, gender, ethnicity, white blood cell, platelet, creatinine, hypertension, anemia, hematologic malignancy, connective tissue disease, peripheral vascular disease, myocardial infarction, liver disease, chronic kidney disease, sepsis, vasopressors, corticosteroids, intravenous fluids, red blood cell transfusions, SOFA, and SAPSII.
Figure 2.
Kaplan-Meier curves for high and low RHR groups before and after propensity score matching (PSM). (A): Kaplan-Meier curves for high and low RHR groups before PSM. (B): Kaplan-Meier curves for high and low RHR groups after PSM.
Figure 3.
Receiver operating characteristic (ROC) curves for RHR, SOFA, SAPSII, RHR-SOFA and RHR-SAPSII in both the survival and non-survival groups, accompanied by the p-value indicating the difference between the ROC curves. (A): ROC curves for RHR and SOFA. (B): ROC curves for RHR and SAPSII. (C): ROC curves for combined RHR-SOFA and SOFA alone. (D): ROC curves for combined RHR-SAPSII and SAPSII alone.
Table 6.
Area Under the Curve (AUC), Cut-off Value, Sensitivity, Specificity for Predicting Death in AECOPD Patients by RHR, SOFA, SAPSII, RHR-SOFA, and RHR-SAPSII
| Variables | AUC | 95% CI | Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|
| RHR | 0.624 | 0.585–0.662 | 0.473 | 59.20% | 62.12% |
| SOFA | 0.640 | 0.601–0.677 | 5 | 53.60% | 66.13% |
| SAPSII | 0.688 | 0.650–0.724 | 36 | 76.60% | 54.11% |
| RHR-SOFA | 0.670 | 0.632–0.707 | - | 64.00% | 63.93% |
| RHR-SAPSII | 0.701 | 0.663–0.737 | - | 77.60% | 55.31% |
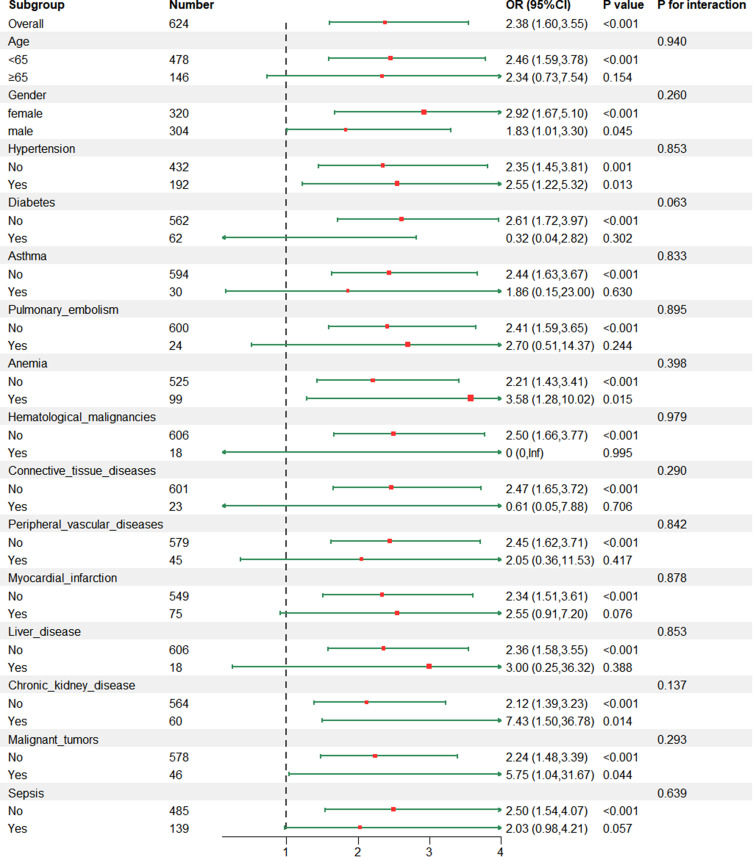
Subgroup Analysis and Sensitivity Analysis
Subgroup analysis was conducted to explore the relationship between RHR and 28-day mortality in different populations, and the findings are presented in Figure 4. The results indicated that there were no significant interactions between RHR and any of the subgroups (P>0.05), demonstrating the stability of RHR. Sensitivity analysis was performed using logistic regression in the eICU database to further evaluate the robustness of RHR. Baseline information for AECOPD patients in the eICU database is documented in Table S4. The logistic regression results are recorded in Table S5, showing that high RHR is significantly positively associated with mortality in AECOPD patients, whether RHR is treated as a continuous or categorical variable.
Figure 4.
The relationship between RHR and 28-days mortality rates of ICU-admitted AECOPD patients in different subgroups.
Discussion
In this retrospective study, we analyzed data from 624 ICU patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) extracted from the MIMIC-IV database. Employing univariate logistic regression, multivariate logistic regression, and propensity score matching, we aimed to mitigate confounding factors. Our findings revealed a noteworthy correlation between elevated high RDW/HCT ratio (RHR) levels and increased 28-day all-cause mortality among ICU patients with AECOPD. Additionally, Kaplan-Meier curves illustrated a significantly lower 28-day survival rate among patients with RHR ≥ 0.473 compared to those with RHR < 0.473, providing further support for our observations. Additionally, the robustness of RHR was validated using a multicenter cohort from the eICU database. Notably, this study marks the first instance where RHR has been identified as an independent predictor of adverse outcomes within 28 days among critically ill AECOPD patients.
Biomarkers are biochemical indicators that reflect the health of organs and tissues and are crucial for early disease detection. They are particularly useful in clinical settings because changes in biomarker levels can often be detected before symptoms and radiological findings become apparent. In COPD, biomarkers play a key role in diagnosis, prognostic evaluations, and treatment management. For example, a large-scale, multicenter, prospective study has shown that early high levels of creatinine are associated with higher hospital mortality rates and increased ICU admissions among emergency department patients with AECOPD.33 Additionally, elevated neutrophil and monocyte counts are early indicators of COPD, which help in timely diagnosis.34 Eosinophil counts are also important for accurately classifying COPD types and guiding the administration of inhaled corticosteroids.35 For the most critically ill COPD patients, especially those with AECOPD in the ICU, early prognostic evaluations and personalized management are vital for effective treatment. The use of biomarkers in this context holds great potential.
We observed that high RHR, calculated as the ratio of red cell distribution width (RDW) to hematocrit (HCT), exhibited comparable performance to the Sequential Organ Failure Assessment (SOFA) score in assessing severity within the ICU setting (area under the curve [AUC]: 0.624 vs 0.640, p=0.663). Although RHR demonstrated lower discriminatory ability compared to the Simplified Acute Physiology Score (SAPS) II, this difference was not statistically significant (p=0.067). Notably, RHR offers the advantage of being easily accessible through routine venous blood tests in clinical practice. Unlike the comprehensive data collection required for SOFA and SAPS II, the RDW/HCT ratio can provide an initial evaluation of AECOPD severity at an earlier stage due to its simplicity and accessibility.
Furthermore, our study highlighted the potential of combining RHR with the SOFA score to enhance prognostic accuracy. The joint application of RHR and SOFA score resulted in a significantly higher AUC area compared to using the SOFA score alone (0.640 vs 0.670, p=0.029). Since the SOFA score does not incorporate RDW or HCT-related indicators, integrating the RDW/HCT ratio with the SOFA score could augment the identification of critically ill AECOPD patients, thus facilitating more precise risk stratification and management decisions.
Kalemci and colleagues have previously noted a correlation between higher RDW levels and the severity of COPD, with RDW exceeding 14.45% associated with severe COPD.36 Similarly, a prospective study by Hu et al identified RDW levels greater than 13.75% as an independent risk factor for in-hospital and one-year mortality in AECOPD patients.14 Consistent with these findings, our study revealed significantly elevated RDW levels in AECOPD patients who succumbed within 28 days in the ICU compared to survivors (p=0.001), with the optimal cutoff value determined as 14.6% based on the receiver operating characteristic (ROC) curve analysis.
Traditionally, it was believed that COPD patients would experience compensatory polycythemia due to hypoxemia resulting from diminished respiratory function, leading to an increase in hematocrit levels. However, recent evidence, including the observations of Quintana et al and Heinemann et al, challenges this notion, suggesting that lower hematocrit levels are associated with greater COPD severity and adverse outcomes.37,38 Consistent with these studies, our findings indicated significantly lower hematocrit levels in AECOPD patients who died within 28 days compared to survivors (p<0.001), with the optimal cutoff value determined as 35.5%.
While there is existing literature on the predictive value of RDW or hematocrit alone, our study investigated the combined ratio of RDW to hematocrit (RHR) as a potential prognostic indicator. Zhu et al reported the superiority of using a combination index of RDW and hemoglobin over individual parameters in identifying frail individuals in the community.39 Additionally, the RDW to hemoglobin ratio has been validated in predicting prognosis in diseases such as sepsis and head and neck cancer.24,25 In line with these observations, our study demonstrated that RHR exhibited a larger AUC area compared to using RDW and HCT alone, albeit without a significant difference observed. Therefore, we elected to utilize RHR as a prognostic indicator for AECOPD patients, recognizing its potential in predicting adverse outcomes.
In our study, we established a cutoff value of 0.473 to classify patients into high and low RHR groups. The high RHR group showed significantly higher mortality compared to the low RHR group (28.1% vs 14.1%, p<0.001). Moreover, even after adjusting for numerous confounding factors using multivariate logistic regression, high RHR levels remained significantly associated with 28-days all-cause mortality in ICU AECOPD patients, both before and after propensity score matching (PSM). Thus, RHR emerges as a stable parameter for assessing 28-days all-cause mortality in ICU AECOPD patients. Further research is warranted to explore the potential differences between RDW/hemoglobin and RDW/HCT and their implications in predicting outcomes in AECOPD patients.
The precise mechanism linking elevated RDW/HCT and increased mortality rates in AECOPD remains elusive. We hypothesize that elevated RDW/HCT, or the derived parameter RHR, may reflect heightened inflammation and oxidative stress levels. During AECOPD, renal cells sense decreased oxygen tension and release erythropoietin (EPO) into the bloodstream.40 EPO stimulates bone marrow stem cell differentiation, erythropoiesis, and the release of immature red blood cells, influencing RDW levels. Although EPO may lead to increased hematocrit, intermittent hypoxemia in COPD patients, along with factors promoting red blood cell destruction, may counteract this effect.41
Inflammation and oxidative stress are key pathogenic mechanisms in COPD, particularly during AECOPD, manifesting as increased circulating inflammatory mediators such as IL-6, TNF-α, and CXCL8.42,43 These cytokines inhibit normal bone marrow red blood cell maturation, leading to the release of immature red blood cells into circulation, thereby increasing RDW.44 Additionally, TNF and IL-1 can directly shorten red blood cell lifespan, lowering hematocrit.45,46 Systemic oxidative stress during AECOPD may disrupt the oxidative-antioxidant balance, activating neutrophils to release reactive oxygen species (ROS) into circulation.47,48 Increased ROS levels may elevate red blood cell lipid peroxidation, affecting cell volume and stiffness, and altering RDW.49 Moreover, systemic oxidative stress may inhibit red blood cell generation, further decreasing hematocrit.50
In summary, AECOPD patients experience a complex interplay of factors leading to red blood cell homeostatic imbalance and metabolic abnormalities, characterized by polychromatophilic erythropoiesis and decreased total red blood cell count.
Despite the insights gained, several limitations exist in this study. Firstly, being a retrospective cohort study, there might be residual confounding factors despite attempts to control known variables. Furthermore, RDW and HCT values were obtained within 24 hours of ICU admission. However, the study primarily focuses on conducting an initial prognosis assessment of ICU patients with AECOPD. In a clinical setting, continuous monitoring of the RDW/HCT ratio allows for a more precise real-time evaluation of patients. In a clinical setting, dynamic monitoring of the RDW/HCT ratio could provide a more accurate real-time assessment of patients. Future prospective multicenter studies are warranted to validate the prognostic utility of RHR in AECOPD patients.
Conclusion
The RDW/HCT ratio can serve as an independent predictor of 28-day all-cause mortality among AECOPD patients in the ICU and assists in the preliminary assessment of patient conditions prior to systematic evaluations. Additionally, the combined use of RHR and the SOFA score exhibits a higher potential for identifying critically ill patients compared to using the SOFA score alone.
Funding Statement
This work was supported by National Key Research and Development Program (2021YFC0864500, 2023YFC3041700), the National Natural Science Foundation of China (82241003), Guangdong Basic and Applied Basic Research Foundation (Grant No.2021A1515110420), Guangzhou Basic and Applied Basic Research Foundation (Grant No.202201010420), and Guangzhou Science and Technology Plans (No. 202201020513).
Abbreviations
RDW, red blood cell distribution width; HCT, hematocrit; COPD, chronic obstructive pulmonary disease; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; ICU, intensive care unit; PSM, propensity score matching; SOFA, sequential organ failure assessment; SAPS II, simplified acute physiology score II; RHR, RDW/HCT ratio.
Data Sharing Statement
The datasets generated and/or analysed during the current study are available in the MIMIC-IV database (https://physionet.org/content/mimiciv/2.2/) and eICU database (https://physionet.org/content/eicu-crd/2.0/).
Consent for Publication
All participants agreed to publish the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no financial or commercial conflict of interest in this work.
References
- 1.Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Am J Respir Crit Care Med. 2023;207(7):819–837. doi: 10.1164/rccm.202301-0106PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruvuna L, Sood A. Epidemiology of chronic obstructive pulmonary disease. Clinics Chest Med. 2020;41(3):315–327. doi: 10.1016/j.ccm.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 3.Stolz D, Mkorombindo T, Schumann DM, et al. Towards the elimination of chronic obstructive pulmonary disease: a lancet commission. Lancet. 2022;400(10356):921–972. doi: 10.1016/s0140-6736(22)01273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thoracic Soc. 2013;10(2):81–89. doi: 10.1513/AnnalsATS.201208-043OC [DOI] [PubMed] [Google Scholar]
- 5.Reis AJ, Alves C, Furtado S, Ferreira J, Drummond M, Robalo-Cordeiro C. COPD exacerbations: management and hospital discharge. Pulmonology. 2018;24(6):345–350. doi: 10.1016/j.pulmoe.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 6.Freund O, Elhadad L, Tiran B, et al. Routine in-hospital interventions during acute exacerbation of COPD are associated with improved 30-day care. Heart Lung. 2024;67:114–120. doi: 10.1016/j.hrtlng.2024.05.001 [DOI] [PubMed] [Google Scholar]
- 7.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: a simple parameter with multiple clinical applications. Critical Rev Clin Lab Sc. 2015;52(2):86–105. doi: 10.3109/10408363.2014.992064 [DOI] [PubMed] [Google Scholar]
- 8.Hu ZD, Chen Y, Zhang L, et al. Red blood cell distribution width is a potential index to assess the disease activity of systemic lupus erythematosus. Int J Clin Chem. 2013;425:202–205. doi: 10.1016/j.cca.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 9.Xu WS, Qiu XM, Ou QS, et al. Red blood cell distribution width levels correlate with liver fibrosis and inflammation: a noninvasive serum marker panel to predict the severity of fibrosis and inflammation in patients with hepatitis B. Medicine. 2015;94(10):e612. doi: 10.1097/md.0000000000000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baba Y, Saito B, Shimada S, et al. Association of red cell distribution width with clinical outcomes in myelodysplastic syndrome. Leukemia Res. 2018;67:56–59. doi: 10.1016/j.leukres.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 11.Deng X, Gao B, Wang F, Zhao MH, Wang J, Zhang L. Red blood cell distribution width is associated with adverse kidney outcomes in patients with chronic kidney disease. Front Med. 2022;9:877220. doi: 10.3389/fmed.2022.877220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Torres V, Royuela A, Múñez-Rubio E, et al. Red blood cell distribution width as prognostic factor in sepsis: a new use for a classical parameter. J Crit Care. 2022;71:154069. doi: 10.1016/j.jcrc.2022.154069 [DOI] [PubMed] [Google Scholar]
- 13.Zhu M, Dai L, Wan L, Zhang S, Peng H. Dynamic increase of red cell distribution width predicts increased risk of 30-day readmission in patients with acute exacerbation of chronic obstructive pulmonary disease. Int J Chronic Obstr. 2021;16:393–400. doi: 10.2147/copd.S291833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu GP, Zhou YM, Wu ZL, et al. Red blood cell distribution width is an independent predictor of mortality for an acute exacerbation of COPD. International J Tuberc Lung Dis. 2019;23(7):817–823. doi: 10.5588/ijtld.18.0429 [DOI] [PubMed] [Google Scholar]
- 15.Luo M, Chen Y, Cheng Y, Li N, Qing H. Association between hematocrit and the 30-day mortality of patients with sepsis: a retrospective analysis based on the large-scale clinical database MIMIC-IV. PLoS One. 2022;17(3):e0265758. doi: 10.1371/journal.pone.0265758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou JK, Zhang QS, Chen YQ, et al. Use of HEMATOCRIT FOR SHORT-TERM PROGNOSIS OF PATIENTS WITH TRAUMATIC BRAIN INJURY AFTER DECOMPRESSIVE CRANIECTomy. World Neurosurg. 2019;123:e141–e146. doi: 10.1016/j.wneu.2018.11.095 [DOI] [PubMed] [Google Scholar]
- 17.Lin JX, Lin JP, Xie JW, et al. Preoperative hematocrit (HCT) is a novel and simple predictive marker for gastric cancer patients who underwent radical gastrectomy. Ann Surg Oncol. 2019;26(12):4027–4036. doi: 10.1245/s10434-019-07582-7 [DOI] [PubMed] [Google Scholar]
- 18.Chambellan A, Chailleux E, Similowski T. Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest. 2005;128(3):1201–1208. doi: 10.1378/chest.128.3.1201 [DOI] [PubMed] [Google Scholar]
- 19.Houben-Wilke S, Jörres RA, Bals R, et al. Peripheral artery disease and its clinical relevance in patients with chronic obstructive pulmonary disease in the COPD and systemic consequences-comorbidities network study. Am J Respir Crit Care Med. 2017;195(2):189–197. doi: 10.1164/rccm.201602-0354OC [DOI] [PubMed] [Google Scholar]
- 20.Zalawadiya SK, Veeranna V, Panaich SS, Afonso L. Red cell distribution width and risk of peripheral artery disease: analysis of national health and nutrition examination survey 1999-2004. Vasc Med. 2012;17(3):155–163. doi: 10.1177/1358863x12442443 [DOI] [PubMed] [Google Scholar]
- 21.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh artery study. Eur Heart J. 2007;28(3):354–362. doi: 10.1093/eurheartj/ehl441 [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi: 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- 23.Jiang W, Zou Z, Zhao S, et al. Erythrocyte transfusion limits the role of elevated red cell distribution width on predicting cardiac surgery associated acute kidney injury. Cardiol J. 2021;28(2):255–261. doi: 10.5603/CJ.a2020.0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tham T, Olson C, Wotman M, et al. Evaluation of the prognostic utility of the hemoglobin-to-red cell distribution width ratio in head and neck cancer. European Archi Oto-Rhino-Laryngol. 2018;275(11):2869–2878. doi: 10.1007/s00405-018-5144-8 [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Chen Z, Yang H, Li H, Chen R, Yu J. Relationship between the hemoglobin-to-red cell distribution width ratio and all-cause mortality in septic patients with atrial fibrillation: based on propensity score matching method. J Cardiovasc Dev Dis. 2022;9(11). doi: 10.3390/jcdd9110400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Wang J. Association between hemoglobin-to-red blood cell distribution width ratio and hospital mortality in patients with non-traumatic subarachnoid hemorrhage. Front Neurol. 2023;14:1180912. doi: 10.3389/fneur.2023.1180912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/bf01709751 [DOI] [PubMed] [Google Scholar]
- 28.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957 [DOI] [PubMed] [Google Scholar]
- 29.Capuzzo M, Moreno RP, Le Gall JR. Outcome prediction in critical care: the simplified acute physiology score models. Current Opinion Critical Care. 2008;14(5):485–490. doi: 10.1097/MCC.0b013e32830864d7 [DOI] [PubMed] [Google Scholar]
- 30.Moreno R, Rhodes A, Piquilloud L, et al. The sequential organ failure assessment (SOFA) score: has the time come for an update? Critical Care. 2023;27(1):15. doi: 10.1186/s13054-022-04290-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson AEW, Bulgarelli L, Shen L, et al. MIMIC-IV, a freely accessible electronic health record dataset. Scientific Data. 2023;10(1):1. doi: 10.1038/s41597-022-01899-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollard TJ, Johnson AEW, Raffa JD, Celi LA, Mark RG, Badawi O. The eICU collaborative research database, a freely available multi-center database for critical care research. Scientific Data. 2018;5:180178. doi: 10.1038/sdata.2018.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freund O, Melloul A, Fried S, et al. Management of acute exacerbations of COPD in the emergency department and its associations with clinical variables. Int Emerg Med. 2024. doi: 10.1007/s11739-024-03592-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapellos TS, Baßler K, Fujii W, et al. Systemic alterations in neutrophils and their precursors in early-stage chronic obstructive pulmonary disease. Cell Rep. 2023;42(6):112525. doi: 10.1016/j.celrep.2023.112525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.David B, Bafadhel M, Koenderman L, De Soyza A. Eosinophilic inflammation in COPD: from an inflammatory marker to a treatable trait. Thorax. 2021;76(2):188–195. doi: 10.1136/thoraxjnl-2020-215167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalemci S, Akin F, Sarihan A, Sahin C, Zeybek A, Yilmaz N. The relationship between hematological parameters and the severity level of chronic obstructive lung disease. Polish Arch Int Med. 2018;128(3):171–177. doi: 10.20452/pamw.4198 [DOI] [PubMed] [Google Scholar]
- 37.Quintana JM, Anton-Ladislao A, Orive M, et al. Predictors of short-term COPD readmission. Int Emerg Med. 2022;17(5):1481–1490. doi: 10.1007/s11739-022-02948-4 [DOI] [PubMed] [Google Scholar]
- 38.Heinemann F, Budweiser S, Jörres RA, et al. The role of non-invasive home mechanical ventilation in patients with chronic obstructive pulmonary disease requiring prolonged weaning. Respirology. 2011;16(8):1273–1280. doi: 10.1111/j.1440-1843.2011.02054.x [DOI] [PubMed] [Google Scholar]
- 39.Zhu M, Wei C, Yang X, Huang Y, Xu Y, Xiong Z. Lower haemoglobin-to-red blood cell distribution width ratio is independently associated with frailty in community-dwelling older adults: a cross-sectional study. BMJ open. 2023;13(7):e069141. doi: 10.1136/bmjopen-2022-069141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27(1):41–53. doi: 10.1016/j.blre.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yčas JW, Horrow JC, Horne BD. Persistent increase in red cell size distribution width after acute diseases: a biomarker of hypoxemia? Int J Clin Chem. 2015;448:107–117. doi: 10.1016/j.cca.2015.05.021 [DOI] [PubMed] [Google Scholar]
- 42.Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7(5):e37483. doi: 10.1371/journal.pone.0037483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–580. doi: 10.1136/thx.2003.019588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paulson RF, Ruan B, Hao S, Chen Y. Stress erythropoiesis is a key inflammatory response. Cells. 2020;9(3). doi: 10.3390/cells9030634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27. doi: 10.1016/j.jaci.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 46.Means RT Jr. Pathogenesis of the anemia of chronic disease: a cytokine-mediated anemia. Stem Cells. 1995;13(1):32–37. doi: 10.1002/stem.5530130105 [DOI] [PubMed] [Google Scholar]
- 47.Noguera A, Batle S, Miralles C, et al. Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax. 2001;56(6):432–437. doi: 10.1136/thorax.56.6.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154(4 Pt 1):1055–1060. doi: 10.1164/ajrccm.154.4.8887607 [DOI] [PubMed] [Google Scholar]
- 49.Joosse HJ, van Oirschot BA, Kooijmans SAA, et al. In-vitro and in-silico evidence for oxidative stress as drivers for RDW. Sci Rep. 2023;13(1):9223. doi: 10.1038/s41598-023-36514-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osawa Y, Tanaka T, Semba RD, et al. Proteins in the pathway from high red blood cell width distribution to all-cause mortality. EBioMedicine. 2022;76:103816. doi: 10.1016/j.ebiom.2022.103816 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the MIMIC-IV database (https://physionet.org/content/mimiciv/2.2/) and eICU database (https://physionet.org/content/eicu-crd/2.0/).