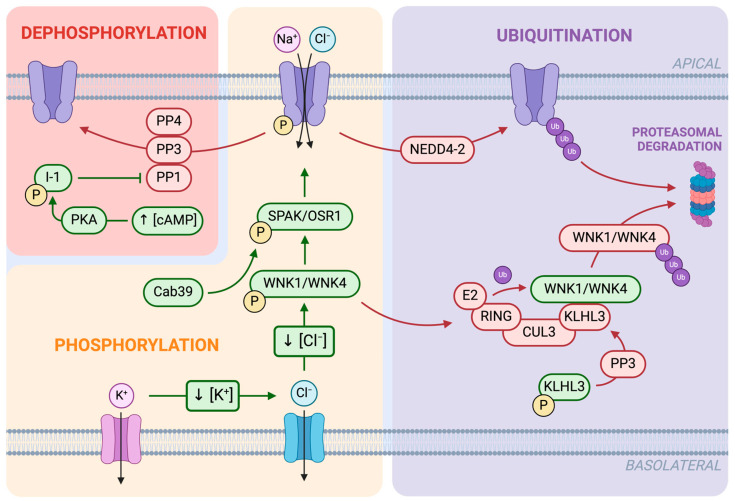
Figure 3.
Post-translational modifications regulating NCC activity in the DCT. NCC phosphorylation (yellow) is primarily mediated by the WNK-SPAK/OSR1 kinase cascade. WNK1 and WNK4 (with-no-lysine kinases) activate SPAK (STE20/SPS1-related proline/alanine-rich kinase) and OSR1 (oxidative stress-response protein 1), which, in turn, phosphorylate the NCC, increasing its transport activity in the apical membrane. WNK autophosphorylation is stimulated by low Cl− intracellular levels, which result from a decrease in K+ intracellular concentration. These changes are, respectively, mediated by basolateral chlorine (light blue) and potassium channels (pink). Cab39 (calcium binding protein 39) is another regulator of NCC phosphorylation since it is required for SPAK activation. NCC dephosphorylation (red) leads to a reduction in its activity and is carried out by protein phosphatases PP1, PP3, and PP4. PP1 is regulated by protein phosphatase 1 inhibitor-1 (I-1) through the cAMP/PKA pathway. A rise in cAMP levels activates PKA (protein kinase A), which phosphorylates I-1, preventing PP1 from dephosphorylating the NCC. Ubiquitination (purple) is another key regulatory mechanism that affects NCC function. WNK abundance is controlled by an E3 ubiquitin ligase complex composed of KLHL3 (Kelch-like protein 3) and CUL3 (Cullin 3). This complex tags the NCC with ubiquitin (Ub), marking it for proteasomal degradation. KLHL3 acts as adapter for WNK, and its phosphorylation prevents this binding. PP3 is responsible for KLHL3 activation through dephosphorylation. Additionally, NEDD4-2, another ubiquitin ligase, directly mediates NCC degradation through ubiquitination. Figure created in BioRender.com [6].