Abstract
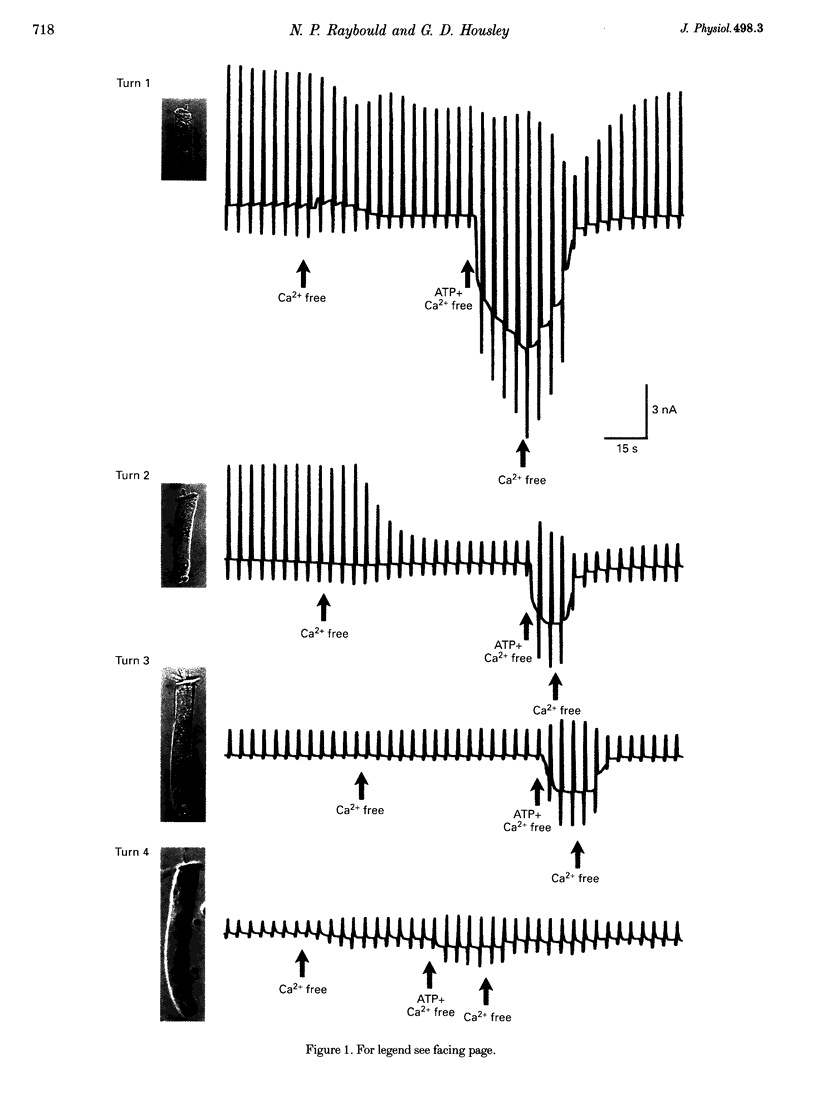
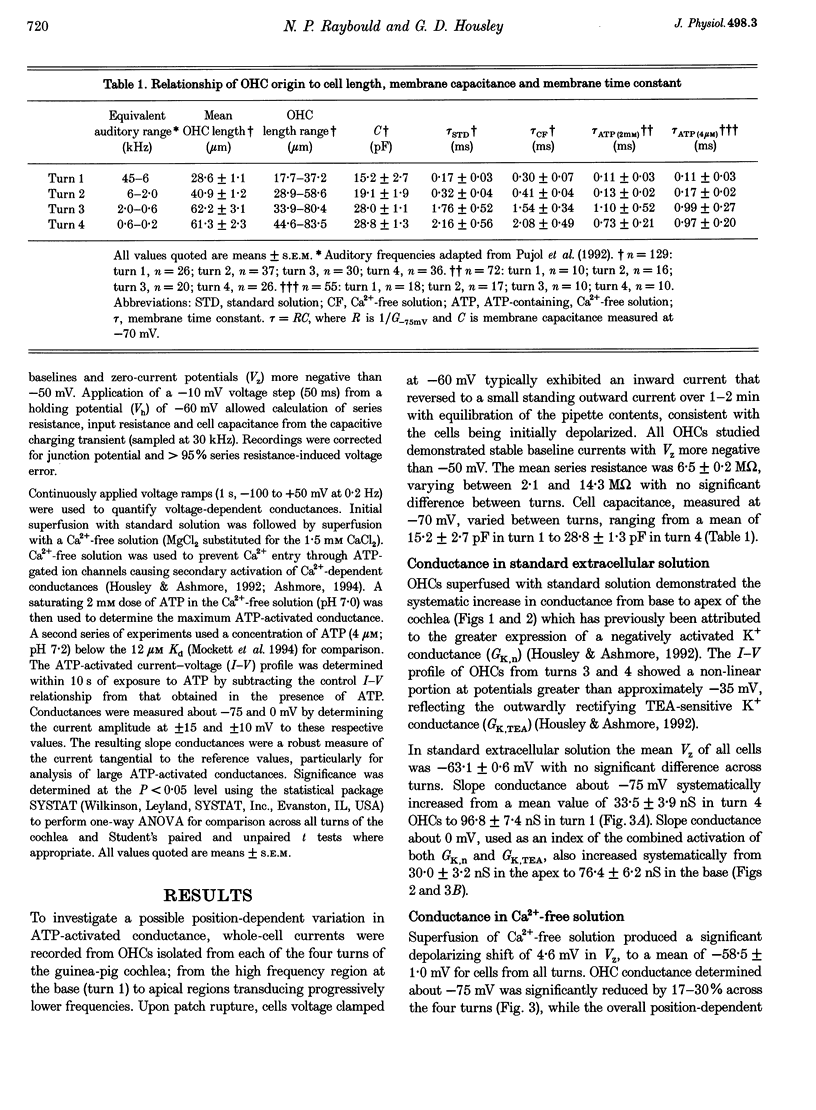
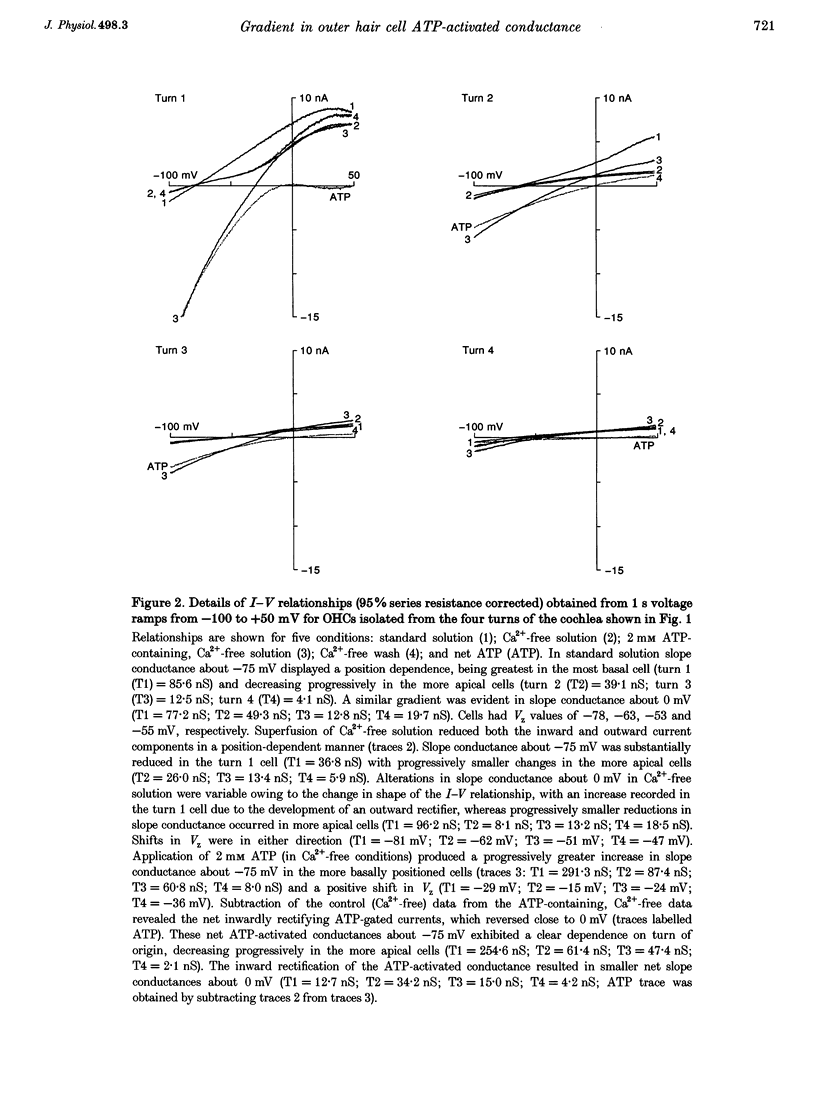
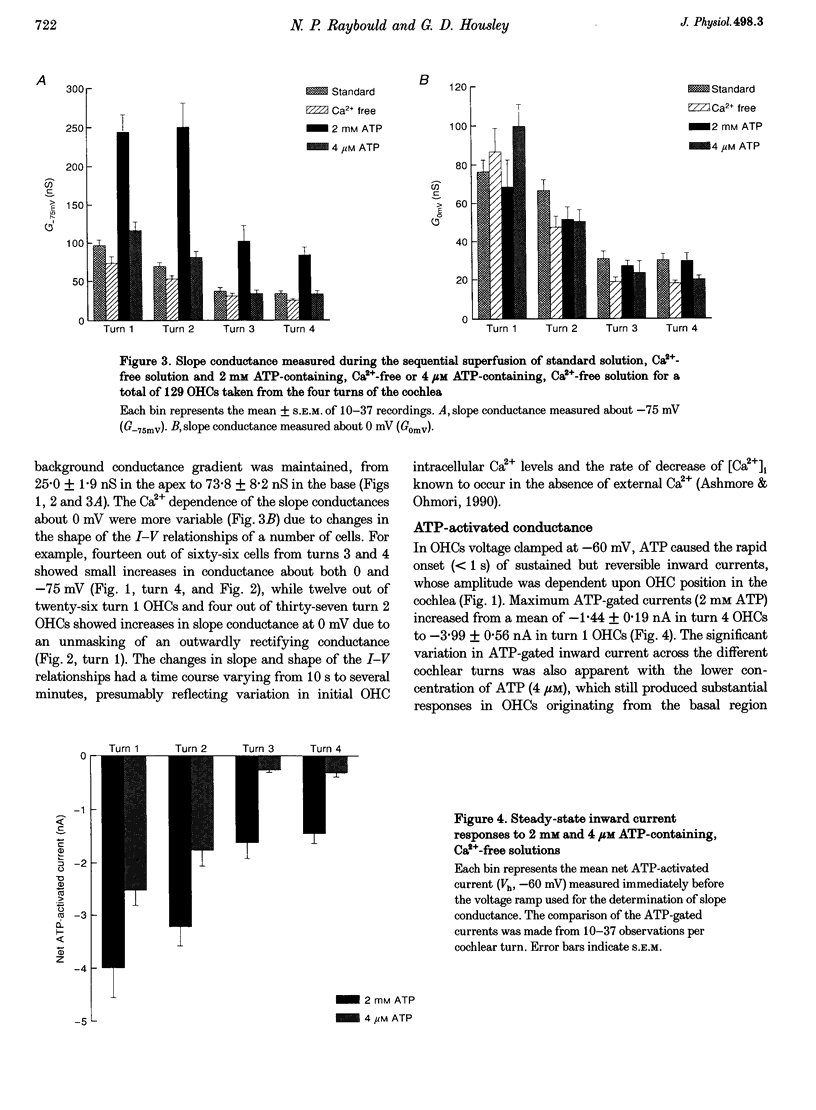
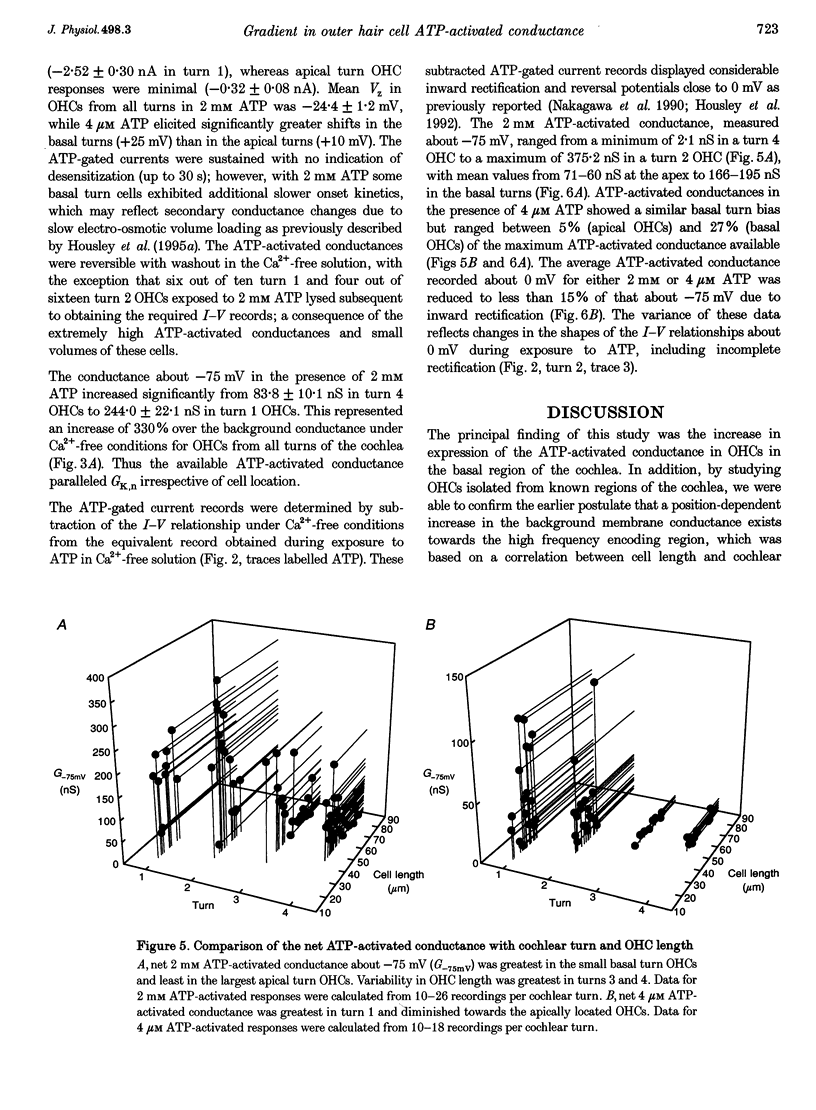
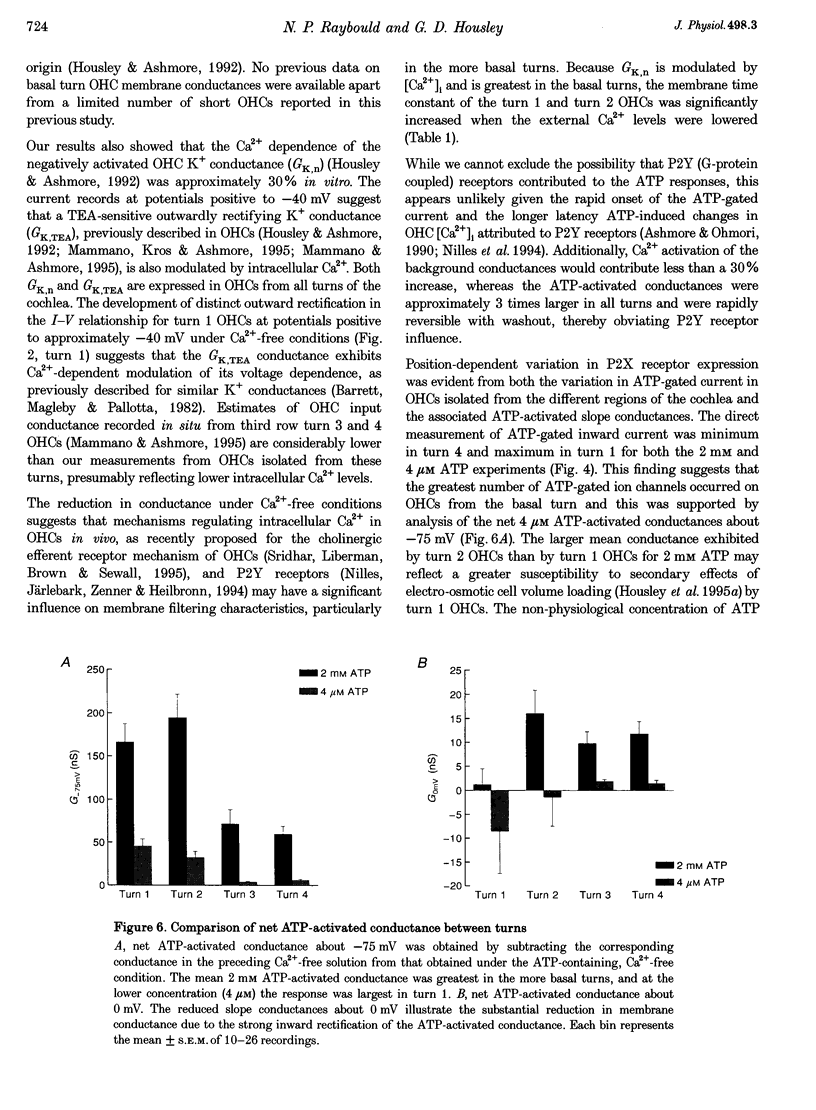
1. Whole-cell patch-clamp recordings were used to determine the variation in the P2X receptor conductance, activated by extracellular ATP, in outer hair cells (OHCs) isolated from each of the four turns of the guinea-pig cochlea. 2. In standard solution (containing 1.5 mM Ca2+) slope conductances were determined in OHCs of known origin from current-voltage relationships obtained from voltage ramps applied between -100 and +50 mV. Membrane conductance throughout this voltage range was greatest in OHCs originating from the basal (high frequency encoding) region of the cochlea. This gradient in OHC conductance from apex to base of the cochlea can be attributed to variation in expression of both a negatively activated K+ conductance and a TEA-sensitive outwardly rectifying K+ conductance. OHC slope conductance measured about a membrane potential of -75 mV increased from a mean of 33.5 nS in the apical region (turn 4) to 96.8 nS in the basal region (turn 1) of the cochlea. 3. Removal of external Ca2+ reduced OHC conductance by an average of 25%, reflecting a Ca2+ dependence of the background conductances in these cells. In zero external Ca2+ the mean slope conductance measured at -75 mV in the apical turn was 25.0 nS compared with 73.8 nS in the basal turn. 4. In Ca(2+)-free solution both 2 mM and 4 microM ATP produced inward currents that were progressively larger in OHCs originating from more basal regions of the cochlea. The steady-state inward current elicited by 2 mM extracellular ATP increased from -1.44 to -3.26 nA for turns 4 and 1, respectively. 5. The P2X receptor conductance was determined between -100 and +50 mV by comparing voltage ramps in the presence and absence of extracellular ATP in Ca(2+)-free solution. The conductance was inwardly rectifying with a reversal potential close to 0 mV. Measured close to the resting membrane potential of the cells (-75 mV), 2 mM ATP elicited an average 300% increase in conductance in parallel with the systematic increase in background conductance which occurs in OHCs originating from the more basal regions of the cochlea. The conductance at -75 mV activated by 2 mM ATP increased from a mean of 59.6 nS in turn 4 OHCs to a mean of 166.2 nS in turn 1 OHCs. The conductance activated by 4 microM ATP was also greater in the basal turn OHCs (45.3 nS) than in the apical region OHCs (5.9 nS). 6. The number of ATP-gated ion channels on individual OHCs, presumed to be localized to the stereocilia, increases from approximately 6000 in turn 4 cells to 16,500 in turn 1 cells, based on estimates of unitary conductance and average maximum ATP-activated OHC conductance (2 mM ATP).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Kolston P. J. Hair cell based amplification in the cochlea. Curr Opin Neurobiol. 1994 Aug;4(4):503–508. doi: 10.1016/0959-4388(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F., Ohmori H. Control of intracellular calcium by ATP in isolated outer hair cells of the guinea-pig cochlea. J Physiol. 1990 Sep;428:109–131. doi: 10.1113/jphysiol.1990.sp018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F. The G.L. Brown Prize Lecture. The cellular machinery of the cochlea. Exp Physiol. 1994 Mar;79(2):113–134. doi: 10.1113/expphysiol.1994.sp003746. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Current state of purinoceptor research. Pharm Acta Helv. 1995 Mar;69(4):231–242. doi: 10.1016/0031-6865(94)00043-u. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. The active cochlea. J Neurosci. 1992 Dec;12(12):4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Housley G. D., Ashmore J. F. Ionic currents of outer hair cells isolated from the guinea-pig cochlea. J Physiol. 1992 Mar;448:73–98. doi: 10.1113/jphysiol.1992.sp019030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley G. D., Greenwood D., Ashmore J. F. Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc Biol Sci. 1992 Sep 22;249(1326):265–273. doi: 10.1098/rspb.1992.0113. [DOI] [PubMed] [Google Scholar]
- Housley G. D., Greenwood D., Bennett T., Ryan A. F. Identification of a short form of the P2xR1-purinoceptor subunit produced by alternative splicing in the pituitary and cochlea. Biochem Biophys Res Commun. 1995 Jul 17;212(2):501–508. doi: 10.1006/bbrc.1995.1998. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J. How the ear's works work. Nature. 1989 Oct 5;341(6241):397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Kujawa S. G., Erostegui C., Fallon M., Crist J., Bobbin R. P. Effects of adenosine 5'-triphosphate and related agonists on cochlear function. Hear Res. 1994 Jun 1;76(1-2):87–100. doi: 10.1016/0378-5955(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Lin X., Hume R. I., Nuttall A. L. Voltage-dependent block by neomycin of the ATP-induced whole cell current of guinea-pig outer hair cells. J Neurophysiol. 1993 Oct;70(4):1593–1605. doi: 10.1152/jn.1993.70.4.1593. [DOI] [PubMed] [Google Scholar]
- Mammano F., Kros C. J., Ashmore J. F. Patch clamped responses from outer hair cells in the intact adult organ of Corti. Pflugers Arch. 1995 Sep;430(5):745–750. doi: 10.1007/BF00386170. [DOI] [PubMed] [Google Scholar]
- Mockett B. G., Bo X., Housley G. D., Thorne P. R., Burnstock G. Autoradiographic labelling of P2 purinoceptors in the guinea-pig cochlea. Hear Res. 1995 Apr;84(1-2):177–193. doi: 10.1016/0378-5955(95)00024-x. [DOI] [PubMed] [Google Scholar]
- Mockett B. G., Housley G. D., Thorne P. R. Fluorescence imaging of extracellular purinergic receptor sites and putative ecto-ATPase sites on isolated cochlear hair cells. J Neurosci. 1994 Nov;14(11 Pt 2):6992–7007. doi: 10.1523/JNEUROSCI.14-11-06992.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz D. J., Thorne P. R., Housley G. D., Billett T. E. Adenosine 5'-triphosphate (ATP) concentrations in the endolymph and perilymph of the guinea-pig cochlea. Hear Res. 1995 Oct;90(1-2):119–125. doi: 10.1016/0378-5955(95)00153-5. [DOI] [PubMed] [Google Scholar]
- Muñoz D. J., Thorne P. R., Housley G. D., Billett T. E., Battersby J. M. Extracellular adenosine 5'-triphosphate (ATP) in the endolymphatic compartment influences cochlear function. Hear Res. 1995 Oct;90(1-2):106–118. doi: 10.1016/0378-5955(95)00152-3. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Akaike N., Kimitsuki T., Komune S., Arima T. ATP-induced current in isolated outer hair cells of guinea pig cochlea. J Neurophysiol. 1990 May;63(5):1068–1074. doi: 10.1152/jn.1990.63.5.1068. [DOI] [PubMed] [Google Scholar]
- Nilles R., Järlebark L., Zenner H. P., Heilbronn E. ATP-induced cytoplasmic [Ca2+] increases in isolated cochlear outer hair cells. Involved receptor and channel mechanisms. Hear Res. 1994 Feb;73(1):27–34. doi: 10.1016/0378-5955(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Sridhar T. S., Liberman M. C., Brown M. C., Sewell W. F. A novel cholinergic "slow effect" of efferent stimulation on cochlear potentials in the guinea pig. J Neurosci. 1995 May;15(5 Pt 1):3667–3678. doi: 10.1523/JNEUROSCI.15-05-03667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa M., Erostegui C., Blanchet C., Dulon D. ATP activates non-selective cation channels and calcium release in inner hair cells of the guinea-pig cochlea. J Physiol. 1996 Mar 15;491(Pt 3):707–718. doi: 10.1113/jphysiol.1996.sp021251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V., Ashmore J. F., Lamb T. D., Menini A. Transduction and adaptation in sensory receptor cells. J Neurosci. 1995 Dec;15(12):7757–7768. doi: 10.1523/JNEUROSCI.15-12-07757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. N., Thorne P. R., Housley G. D., Mockett B., Billett T. E., Burnstock G. Quinacrine staining of marginal cells in the stria vascularis of the guinea-pig cochlea: a possible source of extracellular ATP? Hear Res. 1995 Oct;90(1-2):97–105. doi: 10.1016/0378-5955(95)00151-1. [DOI] [PubMed] [Google Scholar]
- Woolf N. K., Ryan A. F., Harris J. P. Development of mammalian endocochlear potential: normal ontogeny and effects of anoxia. Am J Physiol. 1986 Mar;250(3 Pt 2):R493–R498. doi: 10.1152/ajpregu.1986.250.3.R493. [DOI] [PubMed] [Google Scholar]
- Wright A. Dimensions of the cochlear stereocilia in man and the guinea pig. Hear Res. 1984 Jan;13(1):89–98. doi: 10.1016/0378-5955(84)90099-6. [DOI] [PubMed] [Google Scholar]



