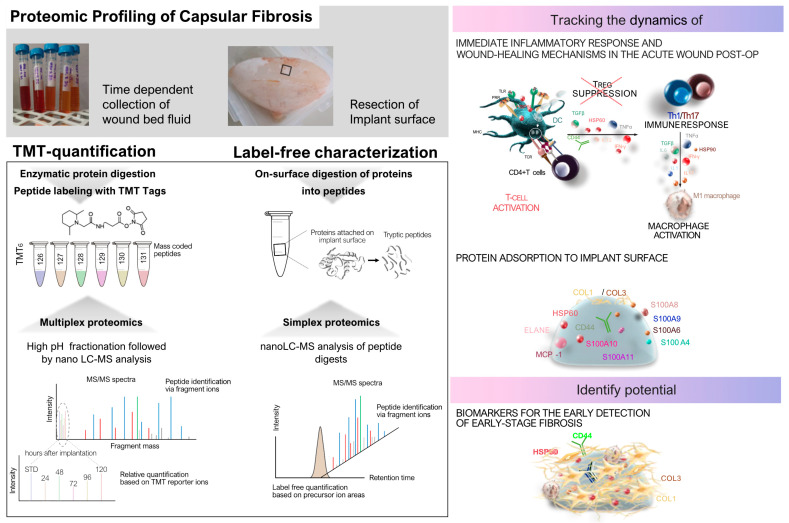
Figure 3.
Proteomic profiling of capsular fibrosis around SMIs. Comprehensive profiling of capsular fibrosis around SMIs through proteomic techniques, elucidating the underlying molecular mechanisms and potential clinical applications. Clinical context: this study comprised 10 female patients who underwent simultaneous prophylactic bilateral nipple-sparing mastectomy (NSME) and tissue expander-based breast reconstruction. Biological samples of wound bed fluid (referred to as WBF) were collected daily from Day 1 to 5 following the expander’s implantation. Wound drains, integral to the surgical procedure for patients undergoing expander-based reconstruction, were retained post-operatively. WBF was collected under sterile conditions. During reoperation, capsular tissue was harvested from two different implants. Proteomic techniques: Time-dependent collection of wound bed fluid and resection of the implant surface followed by enzymatic protein digestion and peptide labeling with TMT tags. Multiplexed proteomics: High pH fractionation, nano-LC-MS analysis of peptide digests, and on-surface digestion of proteins into peptides revealed time-dependent protein expression and quantification post-surgery. Integration and clinical implications. Immediate inflammatory response: This depicts the dynamics of the inflammatory response and wound healing post-implantation, including T cell activation, Th1/Th17 immune response, T regulatory (Treg) cell suppression, and macrophage activation. Protein adsorption and progression of fibrosis: Continuous protein adsorption to the implants’ surface and ECM remodeling contributed to capsular contracture and fibrosis. Biomarkers for early detection: Identified potential biomarkers such as HSPs and S100 proteins for early-stage fibrosis, offering diagnostic and therapeutic insights.