Abstract
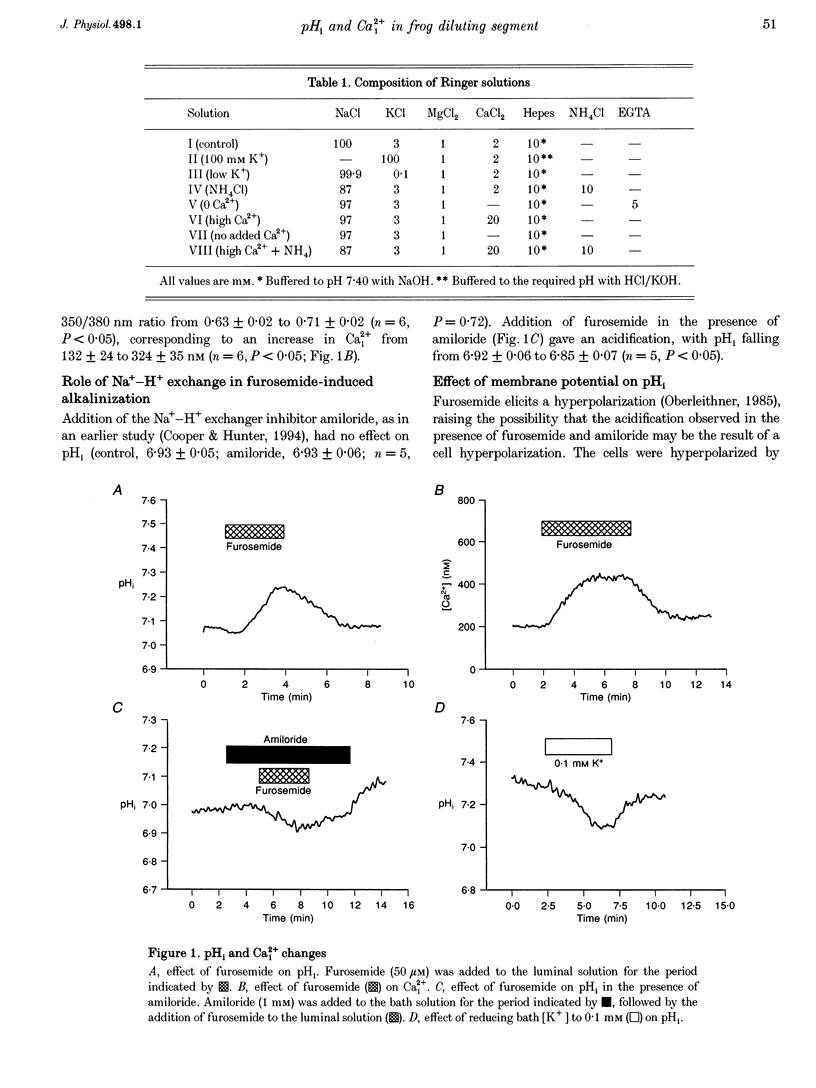
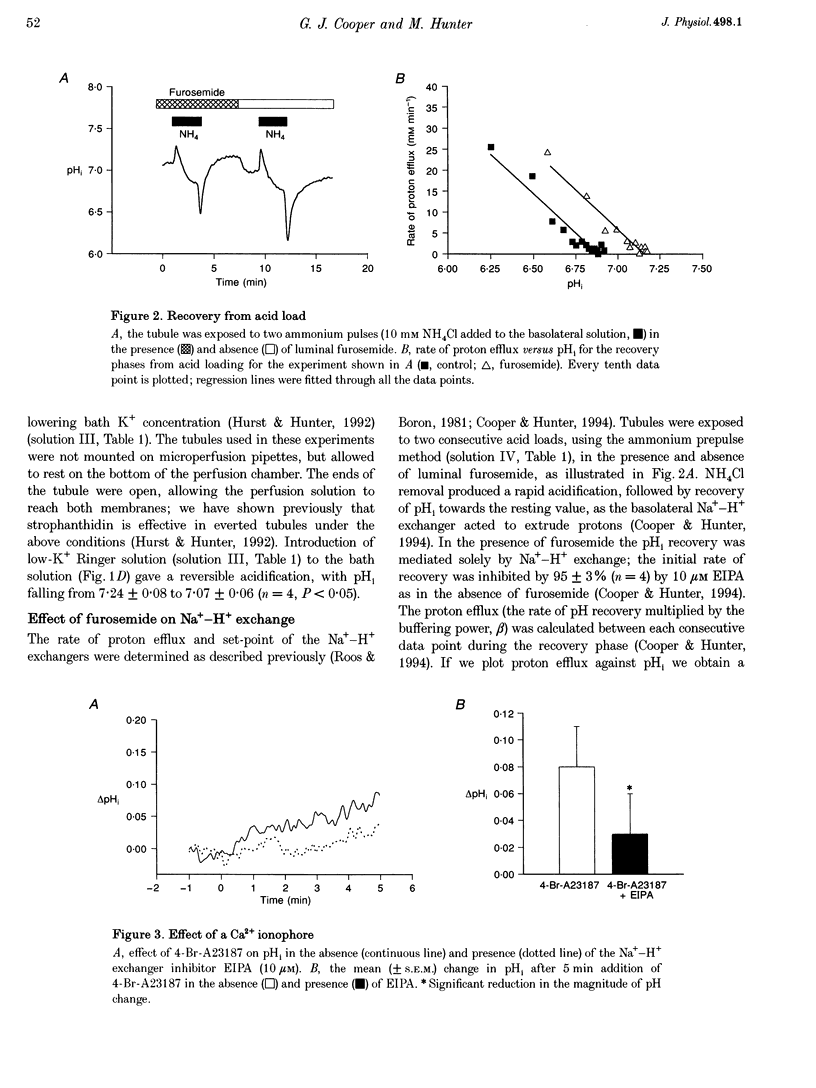
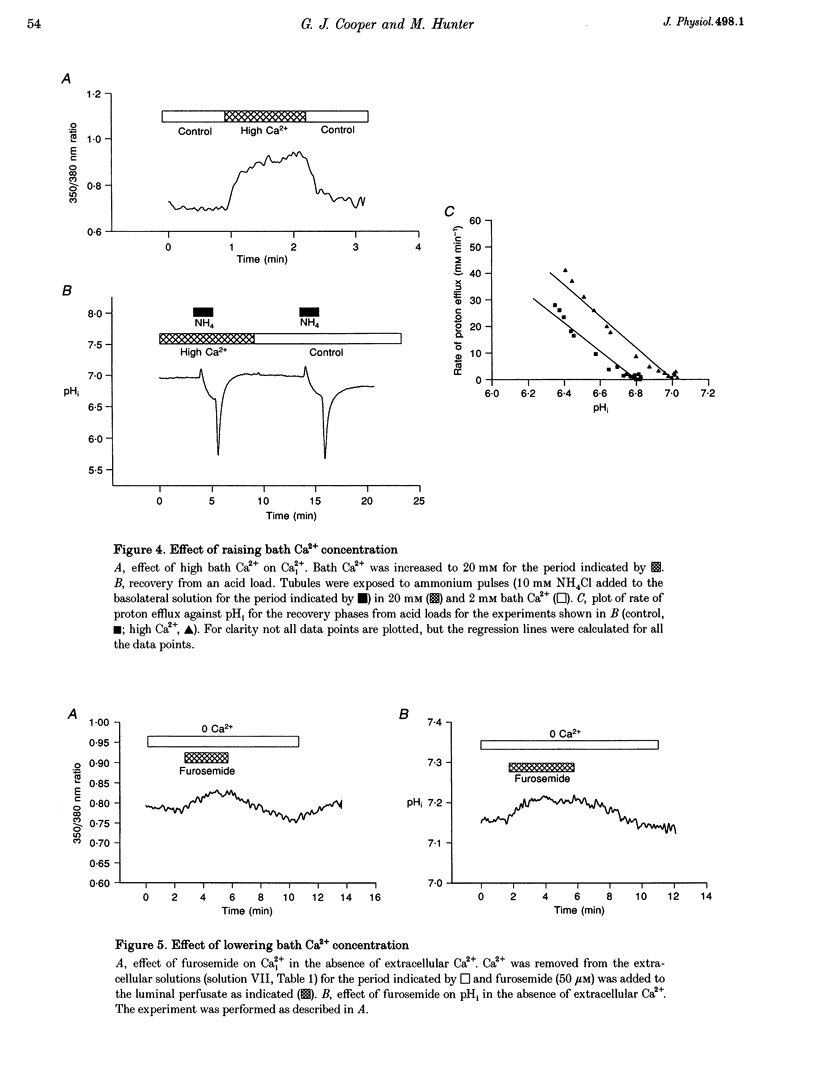
1. The K+ channels of the apical membrane of the diluting segment (early distal tubule, EDT) of the frog are involved in the regulation of transepithelial NaCl transport. These channels are sensitive to pHi and intracellular Ca2+ (Ca2+i). Inhibition of transport by furosemide (frusemide) results in a compensatory increase in K+ channel activity. The aims of the present study were to determine whether pHi or Ca2+i were altered by furosemide, and to identify the means by which such changes were brought about. 2. Experiments were performed using single, microperfused EDT segments. Measurements of pHi and Ca2+i were made using the intracellular fluorescent probes, 2',7'-bis(carboxyethyl)-5,6-carboxyfluorescein (BCECF) and fura-2, respectively. 3. Furosemide increased pHi and Ca2+i. The intracellular alkalinization was the result of an alkaline shift in the set-point of the basolateral Na(+)-H+ exchanger. This response was dependent upon the increase in Ca2+i. 4. The increase in Ca2+i produced by furosemide was due to the release of Ca2+ from intracellular stores. Depletion of these stores, by 2,5-di-t-butylhydroquinone (TBQ) and caffeine, prevented the furosemide-induced changes in Ca2+ and pH. 5. Furosemide-induced activation of Na(+)-H+ exchange was prevented by the calmodulin antagonist, W-7. 6. Thus furosemide elicits a rise in Ca2+i which, via calmodulin, results in activation of Na(+)-H+ exchange. The resulting intracellular alkalinization would be expected to increase channel activity.
Full text
PDF


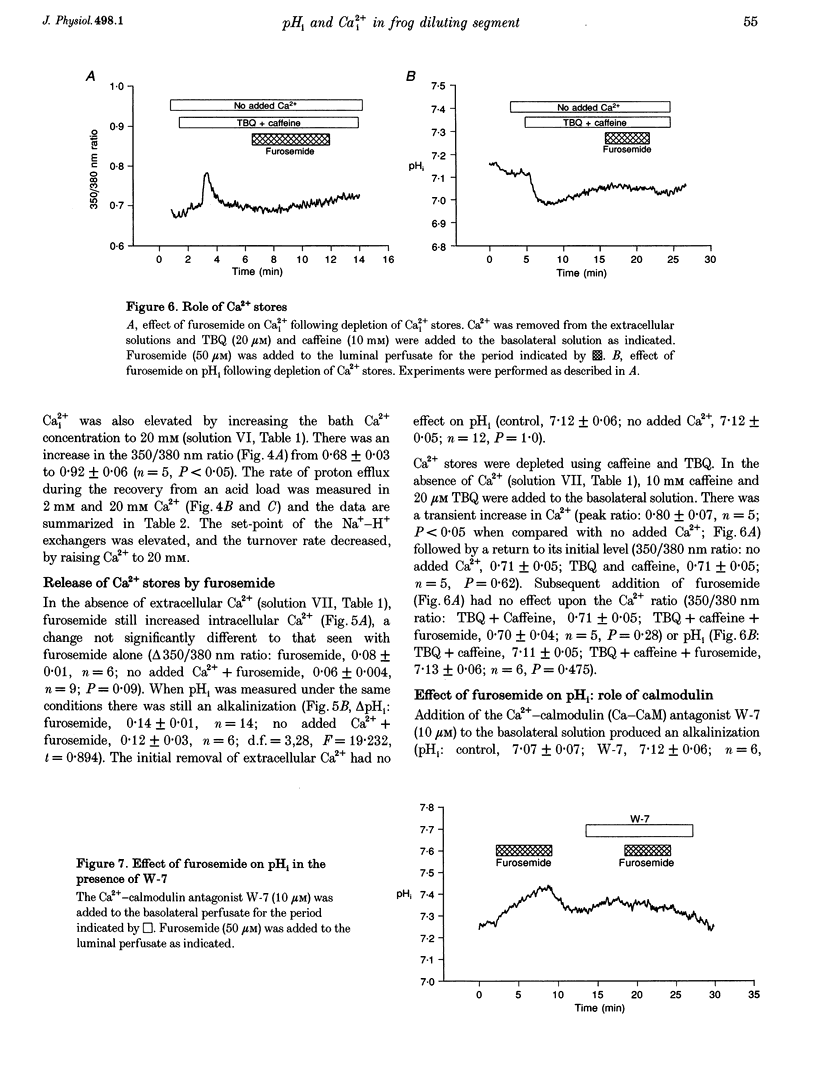


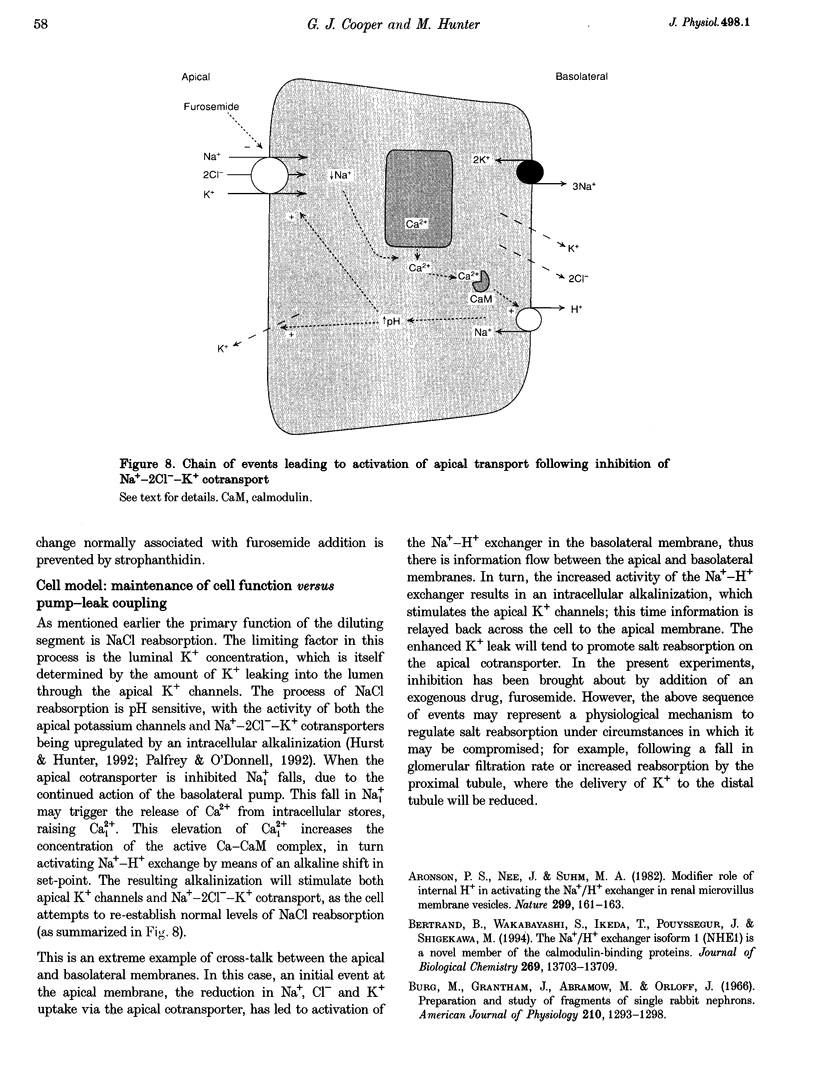

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand B., Wakabayashi S., Ikeda T., Pouysségur J., Shigekawa M. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J Biol Chem. 1994 May 6;269(18):13703–13709. [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Cassel D., Katz M., Rotman M. Depletion of cellular ATP inhibits Na+/H+ antiport in cultured human cells. Modulation of the regulatory effect of intracellular protons on the antiporter activity. J Biol Chem. 1986 Apr 25;261(12):5460–5466. [PubMed] [Google Scholar]
- Cooper G. J., Hunter M. Na(+)-H+ exchange in frog early distal tubule: effect of aldosterone on the set-point. J Physiol. 1994 Sep 15;479(Pt 3):423–432. doi: 10.1113/jphysiol.1994.sp020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. J., Hunter M. Role of de novo protein synthesis and calmodulin in rapid activation of Na(+)-H+ exchange of aldosterone in frog diluting segment. J Physiol. 1996 Feb 15;491(Pt 1):219–223. doi: 10.1113/jphysiol.1996.sp021209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton J. E., Orchard C. H., Boyett M. R. Diastolic, systolic and sarcoplasmic reticulum [Ca2+] during inotropic interventions in isolated rat myocytes. J Physiol. 1991 Jun;437:351–375. doi: 10.1113/jphysiol.1991.sp018600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss G. G., Woodside M., Wakabayashi S., Pouyssegur J., Waddell T., Downey G. P., Grinstein S. ATP dependence of NHE-1, the ubiquitous isoform of the Na+/H+ antiporter. Analysis of phosphorylation and subcellular localization. J Biol Chem. 1994 Mar 25;269(12):8741–8748. [PubMed] [Google Scholar]
- Guggino W. B. Functional heterogeneity in the early distal tubule of the Amphiuma kidney: evidence for two modes of Cl- and K+ transport across the basolateral cell membrane. Am J Physiol. 1986 Mar;250(3 Pt 2):F430–F440. doi: 10.1152/ajprenal.1986.250.3.F430. [DOI] [PubMed] [Google Scholar]
- Hurst A. M., Hunter M. Acute changes in channel density of amphibian diluting segment. Am J Physiol. 1990 Dec;259(6 Pt 1):C1005–C1009. doi: 10.1152/ajpcell.1990.259.6.C1005. [DOI] [PubMed] [Google Scholar]
- Hurst A. M., Hunter M. Apical membrane potassium channels in frog diluting segment: stimulation by furosemide. Am J Physiol. 1992 Apr;262(4 Pt 2):F606–F614. doi: 10.1152/ajprenal.1992.262.4.F606. [DOI] [PubMed] [Google Scholar]
- Lange C. B., Hanke W. Corticosteroid receptors in liver cytosol of the clawed toad, Xenopus laevis: daily and seasonal variations. Gen Comp Endocrinol. 1988 Jul;71(1):141–152. doi: 10.1016/0016-6480(88)90305-x. [DOI] [PubMed] [Google Scholar]
- Oberleithner H. Intracellular pH in diluting segment of frog kidney. Pflugers Arch. 1985 Jul;404(3):244–251. doi: 10.1007/BF00581246. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Lang F., Wang W., Giebisch G. Effects of inhibition of chloride transport on intracellular sodium activity in distal amphibian nephron. Pflugers Arch. 1982 Jul;394(1):55–60. doi: 10.1007/BF01108308. [DOI] [PubMed] [Google Scholar]
- Pouysségur J. Molecular biology and hormonal regulation of vertebrate Na+/H+ exchanger isoforms. Ren Physiol Biochem. 1994 May-Aug;17(3-4):190–193. [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Shen S. S. Na+-H+ antiport during fertilization of the sea urchin egg is blocked by W-7 but is insensitive to K252a and H-7. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1100–1108. doi: 10.1016/0006-291x(89)91356-9. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S., Bertrand B., Ikeda T., Pouysségur J., Shigekawa M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H(+)-sensitive and Ca2+ regulation-defective. J Biol Chem. 1994 May 6;269(18):13710–13715. [PubMed] [Google Scholar]
- Wang W., Dietl P., Silbernagl S., Oberleithner H. Cell membrane potential: a signal to control intracellular pH and transepithelial hydrogen ion secretion in frog kidney. Pflugers Arch. 1987 Jul;409(3):289–295. doi: 10.1007/BF00583478. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]