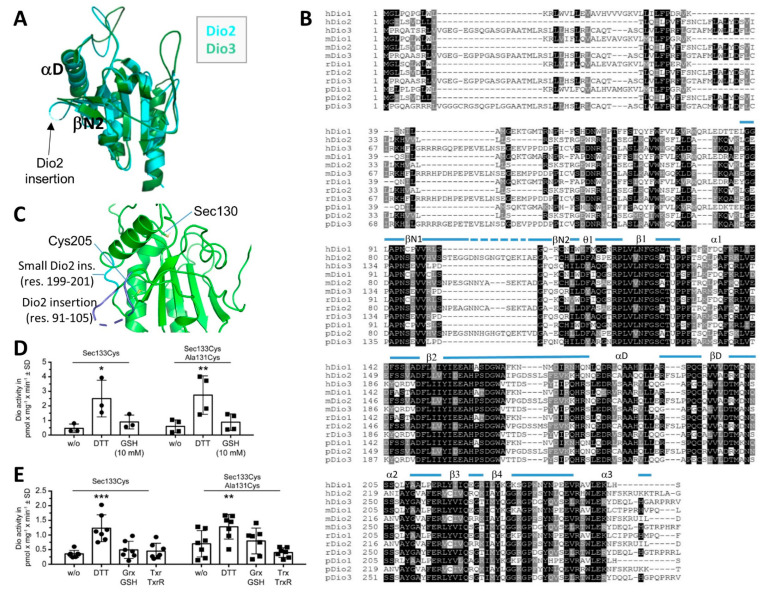
Figure 2.
Dio2-specific structural and catalytic features. (A) Overlay of the crystal structures of the catalytic domains from mouse Dio2 (cyan) and Dio3 (green), respectively. The Dio2-specific insertion is indicated. (B) Sequence alignment for Dio1–3 catalytic domains from human (h), mouse (m), rat (r), and pig (p). The secondary structure elements of mDio2 are illustrated on top (dotted line: not defined by electron density). (C) Crystal structure of Dio2 with the catalytic residue Cys130 (labeled as Sec130), the distal Cys205, and two Dio2-specific insertions indicated (blue). (D) Specific activity of enriched human Dio2(Sec133Cys) after recombinant expression in insect cells; 10 mM GSH did not support deiodination of 125I-rT3, while DTT was able to serve as a reductant. Introduction of a proximal Cys131 neither increased Dio2 activity with DTT nor allowed GSH to serve as a reductant. N = 3–4 independent experiments. * p < 0.05, ** p < 0.01. Two-way ANOVA followed by Dunnett’s t test. (E) Specific activity of enriched human Dio2(Sec133Cys) and Sec133Cys/Ala131Cys recombinantly expressed in insect cells. Neither Grx nor Trx supported deiodination of 125I-rT3, while DTT served as a reductant. N = 7 independent experiments. ** p < 0.01, *** p < 0.001. Two-way ANOVA followed by Dunnett’s t test.