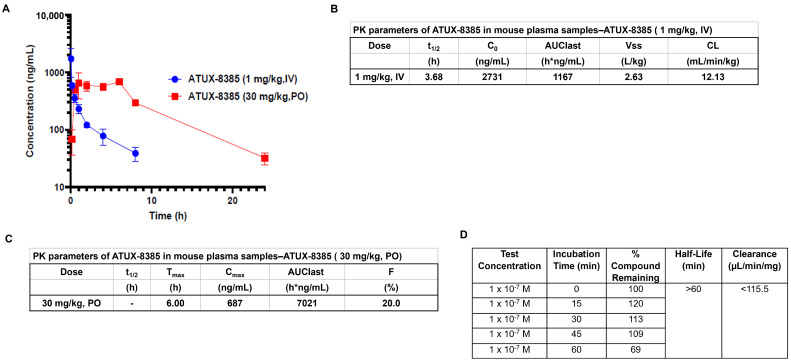
Figure 1.
Pharmacokinetic studies for ATUX-8385. (A–C) Pharmacokinetic studies were completed by Eurofins. ATUX-8385 was administered at 1 mg/kg intravenously (IV) or 30 mg/kg oral (PO) to mice. The plasma samples were collected, processed using acetonitrile precipitation, and analyzed by LC-MS/MS. (A) Plasma concentrations were sustained longer with PO administration. (B,C) Pharmacokinetic data in tabular form for IV and PO dosing of ATUX-8385. (D) Pharmacokinetic data for in vitro metabolism for ATUX-8385. t1/2 = half-life; C0 = concentration at time zero; AUClast = area under the curve from time of administration up to the time of the last quantifiable concentration; Vss = steady-state volume of distribution; CL = clearance; Tmax = time to maximum concentration; Cmax = maximum concentration; F = bioavailability.