Abstract
Background: Early motor development is fundamental in driving cognitive skill acquisition. Motor delays in children with cerebral palsy (CP) often limit exploratory behaviors, decreasing opportunities or the quality of cognitive development, emphasizing the importance of early intervention. This study aimed to assess immediate and 5-month motor and cognitive changes in infants and toddlers at risk of or with CP after participation in a community-based program. Methods: Twenty-two children (mean age: 22 ± 7 months) classified using the Gross Motor Function Classification System (GMFCS) and mini-Manual Ability Classification System (mini-MACS) participated in a 6-day community-based activity program, with outcomes assessed using the Developmental Assessment of Young Children (DAYC-2). Results: Participants who met their motor goals post-participation had significantly higher cognitive scores (p = 0.006) 5 months after the program. Participants with higher functional motor abilities (GMFCS levels I–II, p = 0.052; mini-MACS levels I–II, p = 0.004) demonstrated better cognitive scores at 5 months, adjusted for baseline scores, than those with lower functional motor abilities. Conclusions: This study highlights the impact of motor improvements following an evidence-based community program on later cognitive development. Prospective studies investigating the mechanisms and mediation of cognitive progress in children with CP should investigate the effects of early motor interventions on long-term developmental trajectories.
Keywords: cerebral palsy, motor development, cognition, early intervention
1. Introduction
Motor development in infancy is foundational to cognitive, communicative, social, and emotional development, shaping how children engage with and understand the world around them [1,2,3]. Through motor exploratory activities, infants gather sensory information about objects, test their relationship with the environment, and probe interactions between their bodies, objects, and surroundings, all essential to the development of advanced cognitive abilities [4,5]. For children with motor disorders like cerebral palsy (CP) [6], impairments can limit the opportunity for and quality of exploratory behaviors [4]. As neuroplasticity peaks during the first three years after birth [7], early interventions targeting motor development have the potential to have the greatest effect on long-term developmental trajectories and have been the focus of seminal research [8,9,10,11,12]. Despite a substantial body of interventional studies, there remains a significant gap in research concerning how motor improvements achieved through early interventions influence cognitive development.
Developmental theories like Dynamic Systems Theory (DST) and embodied cognition emphasize the integral contribution of motor skills to cognitive development [13,14,15], and are supported by empirical evidence from neuroimaging [16,17,18] and behavioral studies [19]. The DST views development as a non-linear interaction involving the individual, task, and environment, while embodied cognition asserts that cognitive processes are deeply rooted in physical actions. Neural models like Dynamic Field Theory highlight stable neural activation patterns across sensorimotor brain areas during motor skill acquisition, and suggest a bidirectional relationship where movement fuels cognition, and cognition propels movement [20]. These interactions enhance neural plasticity, with sensorimotor experiences shaping neural pathways and creating cascading effects that enhance both motor and cognitive functions. Early motor impairments can lead to developmental challenges that affect cognitive abilities, making it crucial to identify early motor–cognitive links in children born preterm or with CP to better understand developmental trajectories in these vulnerable groups [21].
To better understand this motor–cognition link, it is essential to categorize motor skills into gross motor skills, involving large muscle groups for actions like walking and jumping, and fine motor skills, which require dexterity for tasks like picking up small objects. Gross and fine motor skills, including manual abilities, are associated with cognitive development in early infancy [22,23,24,25,26,27,28]. Manual abilities encompass dexterity-based tasks that require coordination, precision, and the integration of both gross and fine motor skills [29]. Empirical evidence also suggests that while motor skills support cognitive advancement, cognition reciprocally scaffolds motor mastery [13,15,20,30]. Various aspects of cognition can be tested from executive function to fluid and crystallized intelligence; however, the most common aspect clinically tested in the under-three population is general cognition, encompassing decision-making abilities, functional memory use, play imitation, and purposive planning [29,31,32].
Child development involves a dynamic and interconnected relationship between motor and cognitive skills. Research consistently demonstrates that goal-directed intervention strategies promote motor development in early childhood [10,33]. Specifically, community-based, child-led, and goal-focused interventions and programs have been shown to significantly improve motor and functional outcomes. This approach aligns with the International Classification of Function and Health in Disability (ICF) [34] and its translation into practice using the F-words framework, which emphasizes six key areas: function, family, fitness, fun, friends, and future, all of which prioritize what is meaningful to the child and family [35]. Empowering caregivers with education and support systems not only promotes ongoing developmental progress but also strengthens the caregiver–child bond, which is vital for the child’s overall development [36,37]. As such, the state of Georgia helped fund an evidence-based week-long summer program for families of children at risk of or with CP. In this program, children participated in motor-focused, child-directed, goal-oriented, activities, while family social dynamics and participation were encouraged through a caregiver–child-focused workshop aimed at empowering families. This community program (Climbing Activities Music and Parents, CAMP) also represented the implementation into practice of published clinical principles shown to improve outcomes in this population. By facilitating motor achievements and supporting caregivers, the community CAMP enabled children with CP to reach motor goals while enhancing their parents’ self-efficacy.
This study explored how motor goal attainment may contribute to cognitive development by investigating whether achieving motor goals post-CAMP leads to long-term improvements in cognitive milestones. Using a prospective observational design, we hypothesized that children who achieved their motor goals immediately post-CAMP would have significantly higher cognitive scores at the five-month follow-up. Additionally, we examined how gross motor function and manual ability influenced both immediate post-CAMP goal achievement and five-month motor and cognitive outcomes.
2. Materials and Methods
2.1. Participants
We used a convenience sampling method for participant recruitment. We organized a community activity designed for families of infants and toddlers at risk of or diagnosed with CP. Recruitment spanned the state of Georgia (USA), utilizing various channels such as referrals from ongoing lab studies, Facebook posts, flyers, and direct communication in high-risk infant follow-up programs or community early intervention providers. The activity was available to all families without cost due to state and donor funding, and participation in the associated research study was optional. All families attending CAMP consented to also participate in an observational research study with follow-up, approved by the Institutional Review Board at Emory University (ID—STUDY00006010 and STUDY00006666).
Inclusion criteria comprised children aged 9–36 months (corrected age for those born preterm) with an interim clinical diagnosis of being at high risk of CP or a confirmed CP diagnosis based on established guidelines [38,39]. The two participants designated as high risk for CP received a confirmed CP diagnosis within six months after the conclusion of CAMP. Additionally, caregivers needed to be fluent in English or have access to an interpreter for CAMP activities.
2.2. CAMP Setting
The CAMP program took place in the Baby Brain Optimization Project building in Atlanta, Georgia for three alternating weeks between June and July 2023, with 6–8 families per week. Activities were co-led by a transdisciplinary team, comprising licensed physical, occupational, speech, and music therapists, as well as undergraduate and graduate students from universities across Georgia. Concurrent parent workshops, integral to the program, were developed and conducted by psychologists certified in the Triple P (Positive Parenting Program) [40], social workers, health educators, and case managers. Baseline comprehensive evaluation of CP characteristics, medical and behavioral complexity, and social determinants of health were also performed by the medical team.
In addition to 15–20 min of structured dyadic activities focused on multisensory communication and socialization at the opening and closing of each day, infants and toddlers engaged in two hours of activity across 4 stations with supported breaks to account for child endurance and necessary functions [41]. Participants were paired into groups of two by the therapy team following pre-CAMP assessments, based on similar goals and temperaments [42]. Each station’s workflow aligned to meet initial goals (Table 1).
Table 1.
Example of goal setting: breaking down community and family-centered GAS goals into specific post-CAMP goals for a participant.
| Family GAS Goal | CAMP Goals |
|---|---|
| For my child to be able to walk around the house while holding my hand and to use gestures to express their needs during family meals, in a way that their siblings can understand too, within 4 months | Gross Motor Station: Child will be able to walk 15 feet with moderate assistance around the trunk and will maintain standing while taking support anteriorly from a mirror while playing with sticky toys within 5 days |
| Climbing Station: The child will crawl over a small 15-inch-high tunnel with minimal assistance from the therapist within 5 days | |
| Balance Station: Child will be able to maintain balance while sitting, with minimal support at the trunk, on a dynamic surface, such as a medicine ball, moving side-to-side within 5 days | |
| Picnic and Play: Child will be able to play with the therapist and a peer while waiting for his turn and communicate “more” to the therapist through gestures or words within 5 days |
Goal setting: Caregivers were asked to set a functional, family-centered goal with the CAMP medical team during an initial goal setting session. This included a short- (CAMP) and long-term (4-month) goal in a collaborative process aimed to establish a family-oriented community goal over four months tailored to each family’s needs, aspirations, and the ways they could enhance participation in family life. If the caregiver had difficulty identifying functional goals, then the medical team assisted them through motivational interviewing to identify family-centered goals. This was recorded as the family GAS (Goal Attainment Scale) goal and shared with the CAMP staff during a multidisciplinary team meeting; the 4-month GAS goal was combined with the baseline assessment to establish a measurable post-CAMP goal. While CAMP goals comprise various aspects of the ICF, only motor goals are considered in the current report.
A gross motor station featured a custom gravity-assisted support system (Enliten LLC, Newark, NJ, USA) to aid in mobility skill development by decreasing the effort necessary for anti-gravity skills and decreasing the pressure felt during falls or stumbles [43,44]. Pressure support started at 30% of body weight and was rapidly decreased until weight-bearing and muscle activation efforts were visible. Music therapy was provided with physical therapy to facilitate understanding of rhythmic patterns during ambulatory activities [45,46]. Play-based obstacle courses encouraged postural transition skills and gait adaptations for improving gait stability.
A climbing station targeted coordination, selective motor control, motor planning, and visual motor skills. Activities utilized a toddler-sized rock-climbing wall and modular climbing structures to enhance vestibular, gross motor, and reaching skills [47,48,49]. The station was co-led by an occupational therapist and an adaptive climbing athlete.
A balance station addressed core strengthening and postural stability. The station was led by music therapists with the assistance of physical therapists to design and modify activities. The station incorporated music therapy and toddler-adapted yoga for attunement, socialization, and attention [50,51]. Balance challenges increased with progressive opportunities to experience disequilibrium as trunk control increased.
A picnic and play station focused on fostering communication, manual abilities, social skills, and self-feeding. The station was designed and led by speech therapists and occupational therapists. The station incorporated snack time and play skills, with goals based on the child’s skills and needs.
Circle time: Caregivers participated with their children in a circle time session at the start and end of each CAMP half-day, facilitated by a music therapist. This time was dedicated to music-based caregiver–child engagement, fostering opportunities for bonding, and creating a sense of connection amongst dyads and across the group [52].
Caregiver workshops: While children participated in “station” activities, the caregivers attended a series of support workshops covering how to access caregiver resources in Georgia, how to access therapy services, positive parenting techniques tailored to infants and toddlers with CP, practical tools for promoting mental health and self-care, navigating their child’s healthcare journey, and understanding the ICF model [34]. These efforts supported caregiver involvement and parent-to-parent connection, which has been shown to enhance the success of early interventions [33]. The rationale for incorporating caregiver activities was based on evidence [36] highlighting the need for caregiver education, access to resources, and peer support. Not only does effective caregiver support help build a strong caregiver–child relationship, essential for child development [37], it increases the likelihood that caregivers will engage in positive parenting practices that enhance developmental outcomes [53]. Family GAS goals were incorporated into the workshops to empower caregivers with specificity. On the last day, caregivers received hands-on instruction and coaching from CAMP staff to support their child’s motor goals, along with personalized plans called roadmaps, outlining actionable steps for short-term goal achievement post-CAMP and a customized healthcare roadmap for the year ahead [54,55].
2.3. Measures
Baseline measures, family GAS goals, and CAMP goals were collected on the day before the start of the CAMP activities. Outcomes were measured after 5 days of CAMP. A telehealth follow-up was conducted with families five months later with standardized measures (described below) and a scripted interview aimed at assessing the CAMP experience, feasibility, and acceptability.
Motor and cognitive milestone acquisition was evaluated using the Developmental Assessment of Young Children-2 (DAYC-2) [56] and the cognition and physical development domains via telehealth. The DAYC-2’s suitability for telehealth follow-up was demonstrated during the COVID-19 pandemic [57], as it integrates both caregiver interviews and direct child observation. Due to the brief 6-day interval between baseline and post-CAMP evaluations, raw DAYC-2 scores were used for all analyses rather than norm-referenced scores. The administration of the DAYC-2 was standardized by adapting all questions into lay terms where necessary and ensuring that ceiling-level items were tested according to the standard procedures outlined in the DAYC-2 manual. A video workshop created by the DAYC-2 gold-standard examiner demonstrated proper administration techniques. This was followed by scoring practice with video exams, where all examiners practiced until they achieved over 90% reliability, as verified by the gold-standard examiner. Telehealth administration followed a modified written protocol [57] and was conducted by two DAYC examiners (KB, WK) who had already achieved the required in-person reliability.
The Gross Motor Function Classification System (GMFCS) [58] and mini-Manual Ability Classification System (mini-MACS) [59] were used to classify participants’ motor and manual abilities, respectively. These classifications help in understanding the functional abilities of children with cerebral palsy across different levels. Three experienced therapists jointly assessed and classified all participants after the baseline evaluation to ensure consistent and accurate classification using both GMFCS and mini-MACS levels
Gross motor station goal achievement at the end of CAMP, referred to as post-CAMP motor goals, were recorded as binary data (1 for achievement, 0 for non-achievement). Additionally, the Goal Attainment Scale (GAS) [60] was used to monitor individual short-term goals, termed family GAS goals, targeted for achievement within three to four months. The GAS serves as an individualized metric to quantify progress toward personalized goals, utilizing an ordinal scale that typically ranges from −2 to +2, thereby providing a nuanced measure of goal attainment.
2.4. Statistical Analysis
This observational study compared two groups: those who achieved their motor goal post-CAMP and those who did not. Generalized Linear Mixed Models (GLMMs) were utilized, adjusting for corrected age, with participant ID included as a random effect to account for intra-individual variability. Main effects were further explored using post-hoc within- and between-group comparisons, with effect sizes reported as Cohen’s d [61]. A power analysis for the GLMM, accounting for the correlation of repeated measures, was conducted using G*Power version 3.1.9.6 [62]. With α set to 0.05 and assuming a medium correlation (0.5) and medium effect size ( = 0.33) based on previous literature [25,63,64,65], a post hoc power analysis determined that a sample size of 22 provided 80% power to assess the primary hypothesis of whether post-CAMP goal achievement led to long-term cognitive milestone improvement. These findings are considered robust within the study parameters. Baseline characteristics and GAS goals were compared between the two groups using Wilcoxon rank-sum tests for continuous variables and Fisher’s exact tests for categorical variables. Links between motor and cognitive outcomes were assessed using Spearman rank correlation.
3. Results
Our study cohort included 22 infants and toddlers (mean age = 22 ± 7 months) from a diverse community of children with CP in the state of Georgia (Table 2). The follow-up rate at five months was 100%. At the post-CAMP assessment, 64% of participants met their post-CAMP motor goals, and 86% achieved their four-month family GAS goals. Baseline assessments showed differences in GMFCS and mini-MACS levels between groups who attained their post-CAMP goals and those who did not (Table 3). Developmental raw scores over time are presented in Table 4. At the five-month follow-up, 91% of families rated the CAMP goal-setting workshop as extremely or very helpful, highlighting its positive impact on supporting their children’s developmental goals.
Table 2.
Demographics of Participants.
| Characteristic | Frequency [N = 22] 1 |
|---|---|
| Sex | |
| Females | 9 (41%) |
| Males | 13 (59%) |
| Maternal Education | |
| Less than 7th grade | 1 (4.5%) |
| Partial college or trade school | 3 (13.6%) |
| College graduation | 8 (36.4%) |
| Graduate education | 9 (40.9%) |
| Prefer to not answer | 1 (4.5%) |
| Race | |
| Black/African American | 10 (45.5%) |
| Caucasian | 9 (40.9%) |
| Mixed | 1 (4.5%) |
| Other/Prefer to not answer | 2 (9.0%) |
1 n (%).
Table 3.
Characteristics of CAMP Groups.
| Characteristic | Overall, N (22) 1 |
CAMP Goal Achieved, N (14) 1 |
CAMP Goal Not Achieved, N (8) 1 |
p-Value 2 |
|---|---|---|---|---|
| CA 3 | 22 (7) | 20 (5) | 25 (9) | 0.065 |
| GMFCS | 0.006 * | |||
| Levels I–II | 11 | 10 | 1 | |
| Level III | 5 | 3 | 2 | |
| Levels IV–V | 6 | 1 | 5 | |
| MACS | 0.002 * | |||
| Levels I–II | 12 | 11 | 1 | |
| Level III | 5 | 2 | 3 | |
| Levels IV–V | 5 | 1 | 4 |
1 n; 2 Wilcoxon rank sum exact test; 3 corrected age (CA) reported in months; mean (SD); * statistically significant difference between CAMP goal achieved and goal not achieved groups.
Table 4.
Cognitive and Motor DAYC raw scores between groups from baseline to 5 months.
| Pre-CAMP | Post-CAMP | Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Goal Achieved | Goal Not Achieved | Overall | Goal Achieved | Goal Not Achieved | Overall | Goal Achieved | Goal Not Achieved | |
| Cognition 1 | 24.55 (7.21) | 27.14 (6.13) | 20.00 (7.01) | 25.91 (7.78) | 28.93 (6.75) | 20.63 (6.82) | 29.82 (7.75) | 33.36 (6.25) | 23.63 (6.23) |
|
p-value 2 (Cohen’s d) |
0.036 | 0.013 (1.24) | 0.003 (1.56) | ||||||
| Motor 1 | 39.82 (11.54) | 44.21 (8.12) | 32.13 (13.07) | 40.59 (11.73) | 44.93 (8.36) | 33.00 (13.38) | 44.18 (11.69) | 48.21 (9.10) | 37.13 (12.91) |
|
p-value 2 (Cohen’s d) |
0.040 | 0.040 (1.15) | 0.040 (1.05) | ||||||
1 Mean (SD); 2 between-group comparison (goal achieved vs. goal not achieved) using Wilcoxon rank sum exact test.
3.1. Post-CAMP Motor Goal Success and Long-Term Cognitive Gains
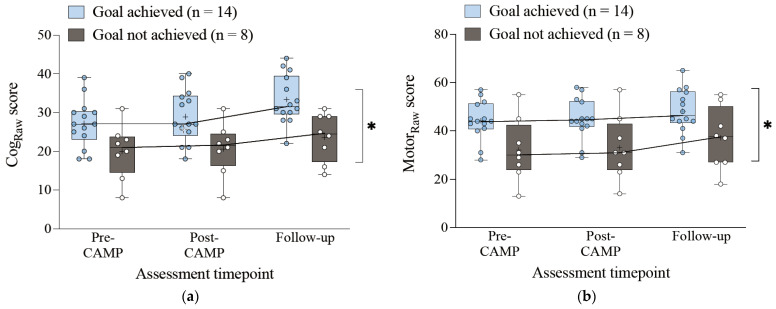
After adjusting for pre-CAMP scores and corrected age in the GLMM, cognitive score changes were higher at the five-month follow-up for participants who met their post-CAMP goals than for those who did not (β = 9.4, p = 0.006) (Table 5). A similar pattern was observed for motor scores (β = 11.9, p = 0.029). Post-hoc analyses revealed statistically significant differences between baseline and post-CAMP scores for those who achieved their motor goals (cognitive: p = 0.004; motor: p = 0.007), but not for those who did not achieve their motor goals (cognitive: p = 0.102; motor: p = 0.059). Statistically significant differences between post-CAMP and five-month follow-up scores were observed for those who achieved their CAMP goals, with moderate to large within-group effect sizes (cognitive: d = 0.67, p = 0.001; motor: d = 0.37, p = 0.008). For those who did not achieve their motor goals, a statistically significant improvement was only observed in the motor domain at follow-up, with moderate effect sizes (cognitive: d = 0.46, p = 0.092; motor: d = 0.31, p = 0.035). Notably, even participants who did not achieve their post-CAMP goals displayed a positive trend in cognitive and motor skill progression at follow-up (Figure 1). Comparing family GAS goal achievement at 5 months between those who achieved their post-CAMP motor goals (100%) and those who did not (63%) did not show statistical significance (p = 0.059).
Table 5.
GLMM for CAMP Goals Group.
| Characteristic | Cognitive | Motor | ||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | |
| Model 1: | ||||||
| CAMP Goal Group * | 0.006 | 0.029 | ||||
| Achieved | 9.4 | 3.0, 15.7 | 11.9 | 1.4, 22.4 | ||
| Time | <0.001 | <0.001 | ||||
| Post | 1.4 | 0.0, 2.7 | 0.8 | −0.5, 2.1 | ||
| Follow Up | 5.3 | 4.0, 6.6 | 4.4 | 3.1, 5.7 | ||
* Group—CAMP goal not achieved was the reference level while controlling for pre-CAMP scores.
Figure 1.
Changes in DAYC-2 raw scores across assessment timepoints, not controlled for age, for (a) cognitive score and (b) motor scores (means are indicated by +). * Statistically Significant differences between groups across all time-points.
3.2. Motor Function Abilities Influencing Cognitive and Motor Outcomes
Preliminary analysis solidified the significant positive correlations between motor function classification (GMFCS, r = 0.48, p = 0.012; mini-MACS, r = 0.53, p = 0.006) and cognitive scores at baseline. Additionally, we found that GMFCS and mini-MACS levels differed significantly at baseline between participants who achieved their post-CAMP goals and those who did not. Participants with higher gross and manual abilities were more likely to achieve their post-CAMP goals, with 91% of those successful classified as level I (Table 3).
We conducted further exploratory analyses examining how GMFCS and mini-MACS levels were associated with cognitive and motor outcomes both immediately post-CAMP and at follow-up (Table 6). In the gross motor model, age-corrected cognitive scores in those at GMFCS level III trended lower than those at GMFCS level I–II (p = 0.052). In the manual ability model, cognitive scores in those at level III were significantly lower (p = 0.004). In both models, participants at levels IV–V had significantly lower cognitive scores compared to the reference group, suggesting that greater motor and manual impairments were linked to poorer cognitive outcomes. Time was also a significant factor, with follow-up scores showing greater improvements in both cognitive and motor outcomes (p < 0.001). Confidence intervals for immediate post-CAMP outcomes included zero, suggesting variability and uncertainty in short-term results (Table 6).
Table 6.
GLMM for GMFCS Groups, and Mini-MACS Groups.
| Characteristic | Cognitive | Motor | ||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | Beta | 95% CI | |||
| Model 2a: | ||||||
| GMFCS Group * | 0.052 | <0.001 | ||||
| Group III | −4.7 | −12.7, 3.3 | −12.7 | −21.8, −3.7 | ||
| Groups IV–V | −8.8 | −17.2, −0.5 | −21.9 | −31.3, 12.5 | ||
| Time | <0.001 | <0.001 | ||||
| Post | 1.4 | 0.0, 2.7 | 0.8 | −0.5, 2.1 | ||
| Follow Up | 5.3 | 4.0, 6.6 | 4.4 | 3.1, 5.7 | ||
| Model 2b: | ||||||
| Mini-MACS Group * | 0.004 | <0.001 | ||||
| Group III | −0.7 | −7.5, −6.0 | −10.2 | −17.9, −2.6 | ||
| Groups IV–V | −12.2 | −18.8, −5.3 | −23.9 | −31.6, −16.3 | ||
| Time | <0.001 | <0.001 | ||||
| Post | 1.4 | 0.0, 2.7 | 0.8 | −0.5, 2.1 | ||
| Follow Up | 5.3 | 4.0, 6.6 | 4.4 | 3.1, 5.7 | ||
* Groups I–II were the reference levels while controlling for pre-CAMP scores.
4. Discussion
Our primary aim was to assess whether achieving short-term motor goals resulted in longer-term cognitive gains in children at risk of or diagnosed with CP. Our results showed that participants who met their motor goals after a one-week community-based program displayed greater improvements in cognitive scores at the five-month follow-up than those who did not. This finding, in children aged three and under with motor impairments characteristic of CP, supports existing research that associates motor gains with cognitive development in this population [21]. It further supports the importance of addressing motor skills during the first three years, as small early motor gains may contribute to larger cognitive and motor improvements in the following months. This is particularly significant since these gains are driven by family-set goals.
Cognitive development in early childhood depends on sensorimotor exploration, where movement refines cognitive processes through sensory feedback and motor planning [5,15,21,32,66]. Sensorimotor experiences play a key role in establishing foundational cognitive skills, such as executive function, which supports higher-level processes like problem solving and planning [16,67,68]. Neuroimaging studies reveal that motor and cognitive processes are interlinked, particularly involving the prefrontal cortex and cerebellum [16,17,18]. These findings emphasize neuroplasticity, where motor experiences during early development reorganize neural pathways and enhance cognitive skills. The disruption of sensorimotor pathways following early brain injury in CP may hinder exploratory behaviors and affect cognitive development [21,69,70]. Early motor gains have been associated with cognitive skill enhancement in children with CP [9]. In our study, the lack of immediate significant changes in cognitive scores after the short-term CAMP program may be attributed either to the brevity of the time interval or to the DAYC-2 assessment tool’s limited sensitivity to detect subtle cognitive changes in the short term [56]. However, effect sizes for within-group comparisons were consistently larger in the cognitive domain compared to motor score progression, indicating positive trends associated with participation in a community-based, child-led, sensorimotor play-based program.
Our analysis of cognitive outcomes across varying levels of gross motor function revealed positive but non-significant trends between GMFCS levels and cognitive scores (p = 0.052), possibly due to sample size and variability. However, when manual abilities were assessed using the mini-MACS, cognitive scores showed significant differences across grouped levels (p = 0.004), suggesting that manual abilities may be a more sensitive predictor of cognitive outcomes than gross motor function [71]. While some studies emphasize the importance of gross motor function in predicting cognitive development [19], it is important to note that hand strength, a key aspect of manual ability, was also assessed in their research. This aligns with literature suggesting that manual skills are particularly crucial for cognitive tasks, as many cognitive assessments rely on fine motor abilities for successful completion [26,72,73]. Our findings are consistent with multiple studies that emphasize a stronger link between manual abilities, fine motor skills [74], and cognitive development in early childhood [22,23,24,25]. Additionally, Cabral et al. [72] demonstrated that in children with CP, manual ability significantly influenced performance on the Bayley Scales of Infant and Toddler Development-III (Bayley-III) cognitive test, especially on tasks requiring fine motor skills. Given the similarities between the Bayley-III and the DAYC-2 scale used in our study [56], this connection may explain the association between manual abilities and cognitive outcomes in our findings.
The primary goal of this study was not to evaluate the effectiveness of the CAMP program, as the observational nature of the design prevents causal attribution. However, we can relate some findings to the context of the existing literature on structured, goal-oriented interventions in community settings for children with motor delays [35,75,76,77]. Following CAMP, 64% of participants met their post-CAMP motor goals. Various studies on short-term interventions have documented motor goal achievements using standardized tools [76,77,78]. For example, an 8-day manual skills-focused camp for school-aged children showed that over 80% of participants achieved their weekly motor goals [79]. This success underscores the dynamic nature of motor skill development and the importance of using longitudinal assessments to assess goal achievement [80,81]. Future prospective studies evaluating the effectiveness of the CAMP as an intervention will provide important information on causal attribution.
Longer periods allow for a more comprehensive understanding of developmental trajectories over time. The 86% success rate in meeting family GAS goals at follow-up highlights the importance of tracking long-term developmental progress and goal achievement within community-based programs. Notably, participants with lower motor function showed delayed but significant improvements in both cognitive and motor abilities at follow-up (Figure 1), and 64% of participants who did not achieve post-CAMP motor goals still met their family GAS goals. This may be attributed to the emphasis on the sustained involvement of empowered caregivers and the educational support provided to families during CAMP. These findings support research identifying community settings as valuable platforms for delivering interventions to children with CP [54,82]. The high rate of goal achievement reflects the effectiveness of these structured approaches, bolstered by caregiver education and peer-to-peer support during community camp. Caregivers reported that 90.9% found the goal-setting workshops helpful. The individualized roadmaps provided ongoing support, enabling families to continue developmental progress post-CAMP.
5. Limitations
In addition to the drawbacks of the assessment tools outlined above, this study had other limitations such as the small sample size reducing the precision and generalizability of the findings, which is reflected in the variability in our data. The absence of a control group further limits definitive conclusions. Our sample also had extreme variability across body function and environmental and personal factors, which was reflected in the results. We acknowledge that the intertwined nature of motor and cognitive development, alongside the multifaceted approach of the CAMP program, complicates pinpointing whether cognitive improvements stemmed from motor gains, enhanced parent–child interactions, or a combination of factors. Future research should explore these mediators to clarify their impact on development. However, this same variability can also present an opportunity. In CP, the relationship between motor and cognitive impairments is complex and non-linear, with only a subset of children experiencing severe cognitive difficulties [83]. This variability, compounded by environment and family factors, allows for the exploration of factors within the relationship between motor skill and cognition that may not be as evident in older children. By investigating early cognitive skill development and providing multisensory and community-based programs that target more than motor systems, this study sought to utilize developmental variability by leveraging a community setting where interventions can be tailored to individual needs.
Given the complex and non-linear nature of developmental trajectories in those with early brain injuries, adaptive methodologies and sensitive assessment tools are required to characterize these evolving trajectories. Future research should incorporate more granular and sensitive measures to track short-term changes in cognitive and motor domains. Adequately powered studies with larger samples and tightly matched controls are needed to isolate intervention effects and provide robust evidence of efficacy. Additionally, single-subject designs could offer deeper insights into individual responses to interventions. While this study employed an intense short-term program, which can be viewed as a limitation, future research should consider exploring longer intervention-based program durations or increased dosage to fully understand the long-term effects. This approach transforms potential limitations into valuable opportunities for refining targeted therapy and research, ultimately optimizing early interventions for individual developmental trajectories.
6. Conclusions
The current study focuses on the links between immediate motor skill improvements following a community-based program and subsequent cognitive progress in infants and toddlers at risk of or diagnosed with CP. The findings suggest that targeting motor abilities can contribute to cognitive gains through evidence-based, goal-directed, multisensory interventions across the ICF domains. While programs like CAMP that are community-based, family-centered, and child-driven in nature offer valuable insights into the motor–cognitive relationship, this study does not aim to guide practices based on the CAMP model as an intervention. Instead, it emphasizes the importance of understanding how motor function influences developmental trajectories, particularly in children with developmental delays. By assessing motor and cognitive outcomes over time, such programs allow researchers to explore how early motor improvements impact broader developmental pathways. This understanding is critical for informing future research on motor–cognitive relationships and developing more comprehensive support strategies for children with developmental delays.
Acknowledgments
We extend our deepest gratitude to all the families who participated in the CAMP program; your commitment and enthusiasm made this study possible. We also wish to sincerely thank the BBOP staff, especially Megan Moran and Paige Ryals, and the students who contributed their time and energy in organizing and conducting the camp; your dedication and support were instrumental in the success of this project.
Author Contributions
Conceptualization, K.B., A.M. and N.L.M.; methodology, K.B., A.M. and N.L.M.; validation, K.B., O.A.K. and N.L.M.; formal analysis, K.B., Z.H. and N.L.M.; investigation, K.B., O.A.K. and Z.H.; resources, N.L.M.; data curation, K.B., A.M., C.P.K., L.R.M., M.M.M., W.C.K., K.L. and N.L.M.; writing—original draft preparation, K.B.; writing—review and editing, K.B., A.M., O.A.K., C.P.K., L.R.M., W.C.K., Z.H., M.M.M., K.L. and N.L.M.; visualization, K.B. and N.L.M.; supervision, N.L.M.; project administration, N.L.M.; funding acquisition, N.L.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Emory University (CAMP protocol ID—STUDY000060100 approved on 20 June 2023; CAMP Follow-up ID—STUDY00006666 approved on 10 October 2023.
Informed Consent Statement
Written informed consent was obtained from all families involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the donation of the Jennings Watkins family trust and through a partnership with the Department of Behavioral Health and Developmental Disabilities to B&B Family Support Services in the State of Georgia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Adolph K.E., Hoch J.E. Motor Development: Embodied, Embedded, Enculturated, and Enabling. Annu. Rev. Psychol. 2019;70:141–164. doi: 10.1146/annurev-psych-010418-102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobo M.A., Galloway J.C. Postural and Object-Oriented Experiences Advance Early Reaching, Object Exploration, and Means–End Behavior. Child. Dev. 2008;79:1869–1890. doi: 10.1111/j.1467-8624.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- 3.Needham A., Barrett T., Peterman K. A pick-me-up for infants’ exploratory skills: Early simulated experiences reaching for objects using ‘sticky mittens’ enhances young infants’ object exploration skills. Infant. Behav. Dev. 2002;25:279–295. doi: 10.1016/S0163-6383(02)00097-8. [DOI] [Google Scholar]
- 4.Babik I., Galloway J.C., Lobo M.A. Early exploration of one’s own body, exploration of objects, and motor, language, and cognitive development relate dynamically across the first two years of life. Dev. Psychol. 2022;58:222–235. doi: 10.1037/dev0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson E.J., Pick A.D. An Ecological Approach to Perceptual Learning and Development. Oxford University Press; New York, NY, USA: 2000. 238p [Google Scholar]
- 6.Graham H.K., Rosenbaum P., Paneth N., Dan B., Lin J.P., Damiano D.L., Becher J.G., Gaebler-Spira D., Colver A., Reddihough D.S., et al. Cerebral palsy. Nat. Rev. Dis. Primers. 2016;2:15082. doi: 10.1038/nrdp.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tierney A.L., Nelson C.A., 3rd Brain Development and the Role of Experience in the Early Years. Zero Three. 2009;30:9–13. [PMC free article] [PubMed] [Google Scholar]
- 8.Dusing S., Harbourne R., Lobo M., McCoy S., Bovaird J. Improvements in motor and cognitive development following sitting together and reaching to play (START-Play): Single subject multiple baseline study of two children. Dev. Med. Child. Neurol. 2018;60:24. doi: 10.1111/dmcn.34_14017. [DOI] [Google Scholar]
- 9.Harbourne R.T., Dusing S.C., Lobo M.A., McCoy S.W., Koziol N.A., Hsu L.-Y., Willett S., Marcinowski E.C., Babik I., Cunha A.B., et al. START-Play Physical Therapy Intervention Impacts Motor and Cognitive Outcomes in Infants With Neuromotor Disorders: A Multisite Randomized Clinical Trial. Phys. Ther. 2021;101:pzaa232. doi: 10.1093/ptj/pzaa232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan C., Fetters L., Adde L., Badawi N., Bancale A., Boyd R.N., Chorna O., Cioni G., Damiano D.L., Darrah J., et al. Early Intervention for Children Aged 0 to 2 Years With or at High Risk of Cerebral Palsy: International Clinical Practice Guideline Based on Systematic Reviews. JAMA Pediatr. 2021;175:846–858. doi: 10.1001/jamapediatrics.2021.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwong A.K., Boyd R.N., Chatfield M.D., Ware R.S., Colditz P.B., George J.M. Early Motor Repertoire of Very Preterm Infants and Relationships with 2-Year Neurodevelopment. J. Clin. Med. 2022;11:1833. doi: 10.3390/jcm11071833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spittle A., Orton J., Anderson P.J., Boyd R., Doyle L.W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. 2015;11:CD005495. doi: 10.1002/14651858.CD005495.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barsalou L.W. Grounded cognition. Annu. Rev. Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- 14.Schöner G., Spencer J.P. Dynamic Thinking: A Primer on Dynamic Field Theory. 1st ed. Oxford University Press; New York, NY, USA: 2016. 399p [Google Scholar]
- 15.Thelen E. Grounded in the World: Developmental Origins of the Embodied Mind. Infancy. 2000;1:3–28. doi: 10.1207/S15327078IN0101_02. [DOI] [PubMed] [Google Scholar]
- 16.Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child. Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- 17.Licea J., Khan O.A., Singh T., Modlesky C.M. Prefrontal cortex hemodynamic activity during a test of lower extremity functional muscle strength in children with cerebral palsy: A functional near-infrared spectroscopy study. Eur. J. Neurosci. 2024;59:298–307. doi: 10.1111/ejn.16211. [DOI] [PubMed] [Google Scholar]
- 18.Koziol L.F., Budding D., Andreasen N., D’Arrigo S., Bulgheroni S., Imamizu H., Ito M., Manto M., Marvel C., Parker K., et al. Consensus Paper: The Cerebellum’s Role in Movement and Cognition. Cerebellum. 2014;13:151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piek J.P., Dawson L., Smith L.M., Gasson N. The role of early fine and gross motor development on later motor and cognitive ability. Hum. Mov. Sci. 2008;27:668–681. doi: 10.1016/j.humov.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Davis M. Movement and Cognition. Theory Pract. 1977;16:207–210. doi: 10.1080/00405847709542700. [DOI] [Google Scholar]
- 21.Babik I., Cunha A.B., Srinivasan S. Biological and environmental factors may affect children’s executive function through motor and sensorimotor development: Preterm birth and cerebral palsy. Infant. Behav. Dev. 2023;73:101881. doi: 10.1016/j.infbeh.2023.101881. [DOI] [PubMed] [Google Scholar]
- 22.Martzog P., Stoeger H., Suggate S. Relations between Preschool Children’s Fine Motor Skills and General Cognitive Abilities. J. Cogn. Dev. 2019;20:443–465. doi: 10.1080/15248372.2019.1607862. [DOI] [Google Scholar]
- 23.Houwen S., Visser L., van der Putten A., Vlaskamp C. The interrelationships between motor, cognitive, and language development in children with and without intellectual and developmental disabilities. Res. Dev. Disabil. 2016;53–54:19–31. doi: 10.1016/j.ridd.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 24.van der Fels I.M.J., te Wierike S.C.M., Hartman E., Elferink-Gemser M.T., Smith J., Visscher C. The relationship between motor skills and cognitive skills in 4–16 year old typically developing children: A systematic review. J. Sci. Med. Sport. 2015;18:697–703. doi: 10.1016/j.jsams.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Cameron C.E., Brock L.L., Murrah W.M., Bell L.H., Worzalla S.L., Grissmer D., Morrison F.J. Fine motor skills and executive function both contribute to kindergarten achievement. Child. Dev. 2012;83:1229–1244. doi: 10.1111/j.1467-8624.2012.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadri F., Sadri I., Krneta Z., Trbojević Jocić J., Batez M. Relationship between cognitive abilities and manual coordination and balance in preschool children. Exerc. Qual. Life. 2021;13:31–38. doi: 10.31382/eqol.210604. [DOI] [Google Scholar]
- 27.Smits-Engelsman B., Hill E.L. The relationship between motor coordination and intelligence across the IQ range. Pediatrics. 2012;130:e950–e956. doi: 10.1542/peds.2011-3712. [DOI] [PubMed] [Google Scholar]
- 28.Veldman S.L.C., Santos R., Jones R.A., Sousa-Sa E., Okely A.D. Associations between gross motor skills and cognitive development in toddlers. Early Hum. Dev. 2019;132:39–44. doi: 10.1016/j.earlhumdev.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Golubović Š., Slavković S. Manual ability and manual dexterity in children with cerebral palsy. Hippokratia. 2014;18:310–314. [PMC free article] [PubMed] [Google Scholar]
- 30.Newell K.M., Liu Y.-T., Mayer-Kress G. A dynamical systems interpretation of epigenetic landscapes for infant motor development. Infant. Behav. Dev. 2003;26:449–472. doi: 10.1016/j.infbeh.2003.08.003. [DOI] [Google Scholar]
- 31.Vygotsky L.S., Cole M. Mind in Society: Development of Higher Psychological Processes. Harvard University Press; Cambridge, MA, USA: 1978. [Google Scholar]
- 32.Piaget J. Part I: Cognitive development in children: Piaget development and learning. J. Res. Sci. Teach. 1964;2:176–186. doi: 10.1002/tea.3660020306. [DOI] [Google Scholar]
- 33.Novak I., McIntyre S., Morgan C., Campbell L., Dark L., Morton N., Stumbles E., Wilson S.-A., Goldsmith S. A systematic review of interventions for children with cerebral palsy: State of the evidence. Dev. Med. Child. Neurol. 2013;55:885–910. doi: 10.1111/dmcn.12246. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization . International Classification of Functioning, Disability, and Health: Children & Youth Version: ICF-CY. World Health Organization; Geneva, Switzerland: 2007. [Google Scholar]
- 35.De Campos A.C., Hidalgo-Robles Á., Longo E., Shrader C., Paleg G. F-words and early intervention ingredients for non-ambulant children with cerebral palsy: A scoping review. Dev. Med. Child Neurol. 2024;66:41–51. doi: 10.1111/dmcn.15682. [DOI] [PubMed] [Google Scholar]
- 36.Dlamini M.D., Chang Y.-J., Nguyen T.T.B. Caregivers’ experiences of having a child with cerebral palsy. A meta-synthesis. J. Pediatr. Nurs. 2023;73:157–168. doi: 10.1016/j.pedn.2023.08.026. [DOI] [PubMed] [Google Scholar]
- 37.Dickinson C., Sheffield J., Mak C., Boyd R.N., Whittingham K. When a baby is diagnosed at high risk of cerebral palsy: Understanding and meeting parent need. Disabil. Rehabil. 2023;45:4016–4024. doi: 10.1080/09638288.2022.2144491. [DOI] [PubMed] [Google Scholar]
- 38.Novak I., Morgan C., Adde L., Blackman J., Boyd R.N., Brunstrom-Hernandez J., Cioni G., Damiano D., Darrah J., Eliasson A.-C., et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017;171:897–907. doi: 10.1001/jamapediatrics.2017.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maitre N.L., Byrne R., Duncan A., Dusing S., Gaebler-Spira D., Rosenbaum P., Winter S. “High-risk for cerebral palsy” designation: A clinical consensus statement. J. Pediatr. Rehabil. Med. 2022;15:165–174. doi: 10.3233/PRM-220030. [DOI] [PubMed] [Google Scholar]
- 40.Sanders M.R., Kirby J.N., Tellegen C.L., Day J.J. The Triple P-Positive Parenting Program: A systematic review and meta-analysis of a multi-level system of parenting support. Clin. Psychol. Rev. 2014;34:337–357. doi: 10.1016/j.cpr.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Morgan C., Darrah J., Gordon A.M., Harbourne R., Spittle A., Johnson R., Fetters L. Effectiveness of motor interventions in infants with cerebral palsy: A systematic review. Dev. Med. Child. Neurol. 2016;58:900–909. doi: 10.1111/dmcn.13105. [DOI] [PubMed] [Google Scholar]
- 42.Palisano R.J., Chiarello L.A., King G.A., Novak I., Stoner T., Fiss A. Participation-based therapy for children with physical disabilities. Disabil. Rehabil. 2012;34:1041–1052. doi: 10.3109/09638288.2011.628740. [DOI] [PubMed] [Google Scholar]
- 43.Prosser L.A., Pierce S.R., Dillingham T.R., Bernbaum J.C., Jawad A.F. iMOVE: Intensive Mobility training with Variability and Error compared to conventional rehabilitation for young children with cerebral palsy: The protocol for a single blind randomized controlled trial. BMC Pediatr. 2018;18:329. doi: 10.1186/s12887-018-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prosser L.A., Pierce S.R., Skorup J.A., Paremski A.C., Alcott M., Bochnak M., Ruwaih N., Jawad A.F. Motor training for young children with cerebral palsy: A single-blind randomized controlled trial. Dev. Med. Child Neurol. 2023;66:233–243. doi: 10.1111/dmcn.15729. [DOI] [PubMed] [Google Scholar]
- 45.Booth A.T.C., Buizer A.I., Meyns P., Oude Lansink I.L.B., Steenbrink F., van der Krogt M.M. The efficacy of functional gait training in children and young adults with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018;60:866–883. doi: 10.1111/dmcn.13708. [DOI] [PubMed] [Google Scholar]
- 46.Kwak E.E. Effect of Rhythmic Auditory Stimulation on Gait Performance in Children with Spastic Cerebral Palsy. J. Music Ther. 2007;44:198–216. doi: 10.1093/jmt/44.3.198. [DOI] [PubMed] [Google Scholar]
- 47.Gassner L., Dabnichki P., Langer A., Pokan R., Zach H., Ludwig M., Santer A. The therapeutic effects of climbing: A systematic review and meta-analysis. PM&R. 2023;15:1194–1209. doi: 10.1002/pmrj.12891. [DOI] [PubMed] [Google Scholar]
- 48.Schram Christensen M., Jensen T., Voigt C.B., Nielsen J.B., Lorentzen J. To be active through indoor-climbing: An exploratory feasibility study in a group of children with cerebral palsy and typically developing children. BMC Neurol. 2017;17:112. doi: 10.1186/s12883-017-0889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kock H., Peixoto G., Labronici R., Oliveira N., Alfieri F., Portes L. Therapeutic climbing: A possibility of intervention for children with cerebral palsy. Acta Fisiatr. 2015;22:35–38. doi: 10.5935/0104-7795.20150008. [DOI] [Google Scholar]
- 50.Mak C., Whittingham K., Cunnington R., Boyd R.N. Effect of mindfulness yoga programme MiYoga on attention, behaviour, and physical outcomes in cerebral palsy: A randomized controlled trial. Dev. Med. Child Neurol. 2018;60:922–932. doi: 10.1111/dmcn.13923. [DOI] [PubMed] [Google Scholar]
- 51.Mössler K., Schmid W., Aßmus J., Fusar-Poli L., Gold C. Attunement in Music Therapy for Young Children with Autism: Revisiting Qualities of Relationship as Mechanisms of Change. J. Autism Dev. Disord. 2020;50:3921–3934. doi: 10.1007/s10803-020-04448-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brecht C.J., Shaw R.J., St. John N.H., Horwitz S.M. Effectiveness of therapeutic and behavioral interventions for parents of low-birth-weight premature infants: A review. Infant. Ment. Health J. 2012;33:651–665. doi: 10.1002/imhj.21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization. United Nations Children’s Fund . Global Report on Children with Developmental Disabilities: From the Margins to the Mainstream. World Health Organization; Geneva, Switzerland: 2023. [Google Scholar]
- 54.Moss S., Gu X. Home- and Community-Based Interventions for Physical Activity and Early Child Development: A Systematic Review of Effective Strategies. Int. J. Env. Res. Public Health. 2022;19:11968. doi: 10.3390/ijerph191911968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedersen M.R.L., Hansen A.F. Interventions by Caregivers to Promote Motor Development in Young Children, the Caregivers’ Attitudes and Benefits Hereof: A Scoping Review. Int. J. Env. Res. Public Health. 2022;19:11543. doi: 10.3390/ijerph191811543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Judith K.V., Maddox T. Developmental Assessment of Young Children. 2nd ed. PRO-ED; Austin, TX, USA: 2013. (DAYC-2) [Google Scholar]
- 57.Maitre N.L., Benninger K.L., Neel M.L., Haase J.A., Pietruszewski L., Levengood K., Adderley K., Batterson N., Hague K., Lightfoot M., et al. Standardized Neurodevelopmental Surveillance of High-risk Infants Using Telehealth: Implementation Study during COVID-19. Pediatr. Qual. Saf. 2021;6:e439. doi: 10.1097/pq9.0000000000000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palisano R., Rosenbaum P., Walter S., Russell D., Wood E., Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 1997;39:214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 59.Eliasson A.-C., Ullenhag A., Wahlström U., Krumlinde-Sundholm L. Mini-MACS: Development of the Manual Ability Classification System for children younger than 4 years of age with signs of cerebral palsy. Dev. Med. Child Neurol. 2017;59:72–78. doi: 10.1111/dmcn.13162. [DOI] [PubMed] [Google Scholar]
- 60.Steenbeek D., Ketelaar M., Galama K., Gorter J.W. Goal attainment scaling in paediatric rehabilitation: A critical review of the literature. Dev. Med. Child Neurol. 2007;49:550–556. doi: 10.1111/j.1469-8749.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 61.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge; London, UK: 2013. [Google Scholar]
- 62.Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 63.Koziol N.A., Kretch K.S., Harbourne R.T., Lobo M.A., McCoy S.W., Molinini R., Hsu L.-Y., Babik I., Cunha A.B., Willett S.L., et al. START-Play Physical Therapy Intervention Indirectly Impacts Cognition Through Changes in Early Motor-Based Problem-Solving Skills. Pediatr. Phys. Ther. 2023;35:293–302. doi: 10.1097/PEP.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 64.Heineman K.R., Schendelaar P., Van den Heuvel E.R., Hadders-Algra M. Motor development in infancy is related to cognitive function at 4 years of age. Dev. Med. Child Neurol. 2018;60:1149–1155. doi: 10.1111/dmcn.13761. [DOI] [PubMed] [Google Scholar]
- 65.Al-Nemr A., Abdelazeim F. Relationship of cognitive functions and gross motor abilities in children with spastic diplegic cerebral palsy. Appl. Neuropsychol. Child. 2018;7:268–276. doi: 10.1080/21622965.2017.1312402. [DOI] [PubMed] [Google Scholar]
- 66.Hofsten C.V. Action, the foundation for cognitive development. Scand. J. Psychol. 2009;50:617–623. doi: 10.1111/j.1467-9450.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 67.Diamond A. Executive Functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diamond A. Why Improving and Assessing Executive Function Early in Life is Critical. In: Griffin J.A., McCardle P., Freund L.S., editors. Executive Function in Preschool-Age Children: Integrating Measurement, Neurodevelopment, and Translational Research. American Psychological Association; Washington, DC, USA: 2016. pp. 11–43. [Google Scholar]
- 69.Jongbloed-Pereboom M., Janssen A.J.W.M., Steenbergen B., Nijhuis-van der Sanden M.W.G. Motor learning and working memory in children born preterm: A systematic review. Neurosci. Biobehav. Rev. 2012;36:1314–1330. doi: 10.1016/j.neubiorev.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Oudgenoeg-Paz O., Mulder H., Jongmans M.J., van der Ham I.J.M., Van der Stigchel S. The link between motor and cognitive development in children born preterm and/or with low birth weight: A review of current evidence. Neurosci. Biobehav. Rev. 2017;80:382–393. doi: 10.1016/j.neubiorev.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 71.Kim H., Carlson A.G., Curby T.W., Winsler A. Relations among motor, social, and cognitive skills in pre-kindergarten children with developmental disabilities. Res. Dev. Disabil. 2016;53–54:43–60. doi: 10.1016/j.ridd.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 72.Invencao Cabral T., Pan X., Tripathi T., Ma J., Heathcock J.C. Manual Abilities and Cognition in Children with Cerebral Palsy: Do Fine Motor Skills Impact Cognition as Measured by the Bayley Scales of Infant Development? Behav. Sci. 2023;13:542. doi: 10.3390/bs13070542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dellatolas G., De Agostini M., Curt F., Kremin H., Letierce A., Maccario J., Lellouch J. Manual skill, hand skill asymmetry, and cognitive performances in young children. Laterality. 2003;8:317–338. doi: 10.1080/13576500342000121. [DOI] [PubMed] [Google Scholar]
- 74.Duncan A.F., Bann C.M., Maitre N.L., Peralta-Carcelen M., Hintz S.R. Hand Function at 18–22 Months Is Associated with School-Age Manual Dexterity and Motor Performance in Children Born Extremely Preterm. J. Pediatr. 2020;225:51–57.e53. doi: 10.1016/j.jpeds.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Butzman B.N., Lau C., Vanier C. Aquatic developmental play program for children in early intervention: A case series. Open J. Occup. Ther. 2022;10:1–18. doi: 10.15453/2168-6408.1833. [DOI] [Google Scholar]
- 76.Dunford C. Goal-orientated group intervention for children with developmental coordination disorder. Phys. Occup. Ther. Pediatr. 2011;31:288–300. doi: 10.3109/01942638.2011.565864. [DOI] [PubMed] [Google Scholar]
- 77.Araneda R., Klöcker A., Ebner-Karestinos D., Sogbossi E.S., Renders A., Saussez G., Paradis J., Bleyenheuft Y. Feasibility and effectiveness of HABIT-ILE in children aged 1 to 4 years with cerebral palsy: A pilot study. Ann. Phys. Rehabil. Med. 2021;64:101381. doi: 10.1016/j.rehab.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 78.McNamara L.M., Scott K.M., Boyd R.N., Webb A.E., Taifalos C.J., Novak I.E. Effectiveness of early diagnosis of cerebral palsy guideline implementation: A systematic review. Minerva Pediatr. 2024;76:414–424. doi: 10.23736/S2724-5276.22.07112-9. [DOI] [PubMed] [Google Scholar]
- 79.Bono G.L.P., Achermann P., Rückriem B., Lieber J., van Hedel H.J.A. Goal-Directed Personalized Upper Limb Intensive Therapy (PULIT) for Children With Hemiparesis: A Retrospective Analysis. Am. J. Occup. Ther. 2022;76:7606205050. doi: 10.5014/ajot.2022.049008. [DOI] [PubMed] [Google Scholar]
- 80.Bain K., Bombria S.D., Chapparo C.J., Donelly M., Heard R., Treacy S. Goal attainment of children with cerebral palsy participating in multi-modal intervention. Child Care Health Dev. 2023;49:1066–1075. doi: 10.1111/cch.13117. [DOI] [PubMed] [Google Scholar]
- 81.Bard-Pondarré R., Villepinte C., Roumenoff F., Lebrault H., Bonnyaud C., Pradeau C., Bensmail D., Isner-Horobeti M.E., Krasny-Pacini A. Goal Attainment Scaling in rehabilitation: An educational review providing a comprehensive didactical tool box for implementing Goal Attainment Scaling. J. Rehabil. Med. 2023;55:jrm6498. doi: 10.2340/jrm.v55.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maulik P.K., Darmstadt G.L. Community-based interventions to optimize early childhood development in low resource settings. J. Perinatol. 2009;29:531–542. doi: 10.1038/jp.2009.42. [DOI] [PubMed] [Google Scholar]
- 83.Stadskleiv K. Cognitive functioning in children with cerebral palsy. Dev. Med. Child Neurol. 2020;62:283–289. doi: 10.1111/dmcn.14463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.