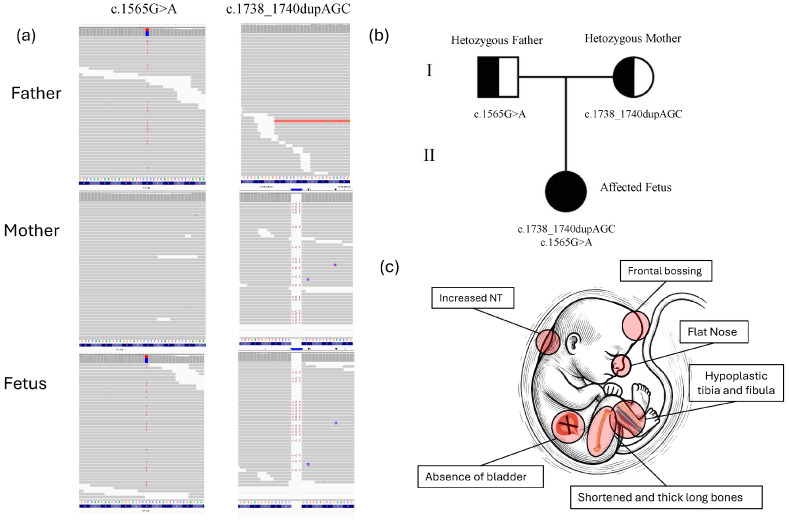
Figure 2.
Prenatal ultrasound findings of fetus with skeletal ciliopathies. (a) Using fetal DNA obtained through amniocentesis, a normal karyotype and the absence of pathogenic copy number variants (CNVs) by array comparative genomic hybridization (aCGH) were assessed. Clinical exome sequencing (CES) and bioinformatic analysis revealed compound heterozygous mutations in the IFT140 gene. The heterozygous IFT140 variant c.1738_1740dupAGC (p.Ser580dup) in one allele and a heterozygous IFT140 variant c.1565G>A (p.Gly522Glu) in the second allele, maternally and paternally inherited, respectively (Figure 2). Both variants were also confirmed by Sanger sequencing in fetus as well as in parents (Figure S1). The missense variant c.1565G>A (p.Gly522Glu) has previously been reported as a likely pathogenic variant in ClinVar (RCV001268554.8) in patients with Sensenbrenner syndrome, Jeune asphyxiating thoracic dystrophy syndrome, Mainzer–Saldino syndrome, and retinitis pigmentosa [3,4,5]. The duplication variant in IFT140 c.1738_1740dupAGC (p. Ser580dup) is classified as a variant of uncertain significance (VUS) in ClinVar (VCV001426203.6) and has not been reported in other public databases, like HGMD Professional (accessed on 10 June 2024), or in individuals affected by IFT140-related conditions. This in-frame duplication results in the insertion of an additional serine amino acid at position 580 within the protein sequence. The insertion occurs in a region of the IFT140 protein that is important for its function, potentially altering the structural integrity and/or the functional dynamics of the protein. Furthermore, the detection of this variant in trans with a likely pathogenic variant, along with its compound heterozygous inheritance from two unaffected parents, provides evidence for its pathogenicity. According to the gnomAD database (as of 25 July 2024), the frequency of the p.Ser580dup variant is 0.000%, indicating it is extremely rare in the general population. This supports the application of ACMG criteria PM2 (extremely low frequency in population databases). Additionally, the compound heterozygous inheritance meets the criteria for PM3, and the observed duplication of a critical amino acid aligns with PM4, further strengthening the classification of the variant as likely pathogenic [6]. (b) Pedigree of the family. Half-filled symbols indicate carrier status for IFT140 variants, roman numerals are used to indicate generations. (c) A minimalistic figure presents the fetus, emphasizing the clinical signs identified in the fetus. In particular, a prenatal ultrasound revealed, in addition to increased nuchal translucency (NT), markedly shortened and thickened long bones, hypoplastic tibia and fibula, suspected absence of the bladder, a flat nasal bridge, and pronounced frontal bossing. These skeletal abnormalities, along with facial dysmorphisms and potential visceral anomalies, strongly suggest a diagnosis consistent with a skeletal ciliopathy, a disorder typically characterized by defects in the structure and function of primary cilia affecting multiple organ systems [7]. The IFT140 gene encodes the IFT140 protein, which is part of a large multi-protein complex known as the intraflagellar transport (IFT) complex. This complex plays a critical role in the formation, maintenance, and function of cilia—hair-like structures that extend from the surface of various cell types. The IFT140 protein is a component of the IFT-A complex, which primarily governs retrograde intraflagellar transport within cilia, facilitating the movement of cargo proteins from the tip of the cilium to its base. The IFT140 gene comprises 31 exons (29 of which are coding) and encodes a protein of 1462 amino acids, featuring five WD repeats and nine tetratricopeptide (TPR) repeats [4,8,9]. Biallelic pathogenic variants in IFT140 or other genes within the IFT-A complex can lead to defective retrograde ciliary transport. Such defects can result in a group of disorders called ciliopathies, which can impact multiple organ systems and cause a wide range of symptoms, including vision and hearing loss, skeletal abnormalities, kidney disease, and developmental delays [7,10]. More than 95 variants in the IFT140 gene have been described in patients with Mainzer–Saldino syndrome or other short-rib thoracic dysplasia phenotypes, according to the HGMD database (2024.2 version). The rarest phenotype associated with IFT140-related ciliopathies is cranioectodermal dysplasia (CED). In the present study, the fetus was characterized by abnormalities of the long bones, hypoplastic tibia and fibula, flat nose, and frontal bossing with involvement of internal organs such as the refereed bladder absence. We characterized a novel heterozygous likely pathogenic variant p.Ser580dup in combination with a known likely pathogenic variant p.Gly522Glu in the IFT140 gene. Additionally, we identified a new clinical feature associated with this phenotype, the absence of the bladder, which had never been previously reported in prenatal diagnosis of skeletal ciliopathies, whereas no other internal organ involvement was observed. The presence of shortened, thickened long bones, hypoplastic tibia and fibula, along with facial features such as frontal bossing and a flattened nose, aligns with the clinical features of short-rib thoracic dysplasia (SRTD), with or without polydactyly [11,12,13]. This group includes Jeune asphyxiating thoracic dystrophy (JATD), short-rib polydactyly syndrome (SRPS), Mainzer–Saldino syndrome (MZSD), and cranioectodermal dysplasia (CED), which exhibit diverse bone and/or cartilage abnormal phenotypes [14]. The identified genotype and ultrasonic skeletal features share several phenotypic features with previously reported patients carrying IFT140 variants, particularly those associated with skeletal ciliopathies such as Mainzer–Saldino syndrome and Sensenbrenner syndrome [3,4,5] Similarly to these cases, our fetus exhibited shortened and thickened long bones, hypoplastic tibia and fibula, and facial dysmorphisms, including a flat nasal bridge and frontal bossing. The absence of the bladder observed in our fetus is a novel finding not previously reported in association with IFT140 mutations or skeletal ciliopathies in general. Previous studies have primarily reported renal anomalies such as cystic kidney disease or nephronophthisis in patients with IFT140 variants [3,5,9]. In conclusion, genetic analysis, such as exome sequencing, is crucial in the prenatal diagnosis of skeletal ciliopathies. These conditions have overlapping clinical features, making differentiation challenging through ultrasound alone. Identifying genetic variants in genes like IFT140 helps clarify the diagnosis, guide prognosis, and inform genetic counseling for affected families, providing critical insights for future pregnancy management. Reporting additional cases of fetal skeletal ciliopathies will aid in identifying genotype-phenotype correlations and pave the way for more accurate clinical diagnosis in prenatal settings.