Abstract
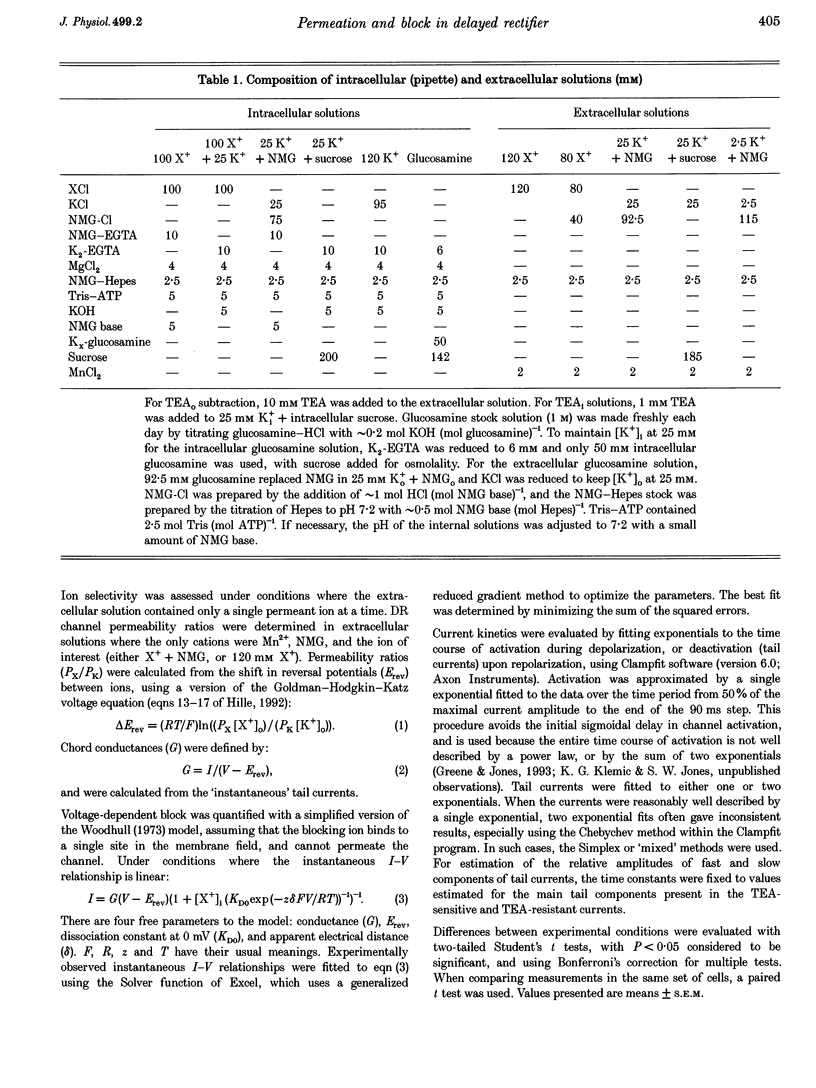
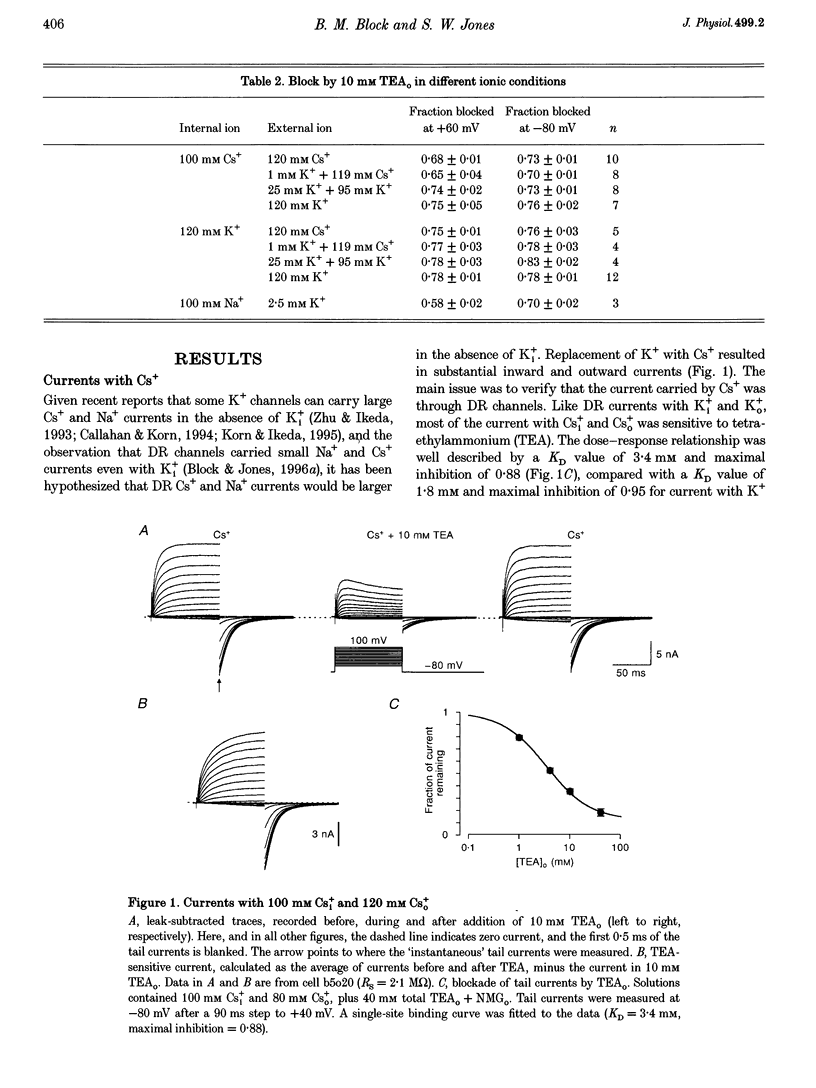
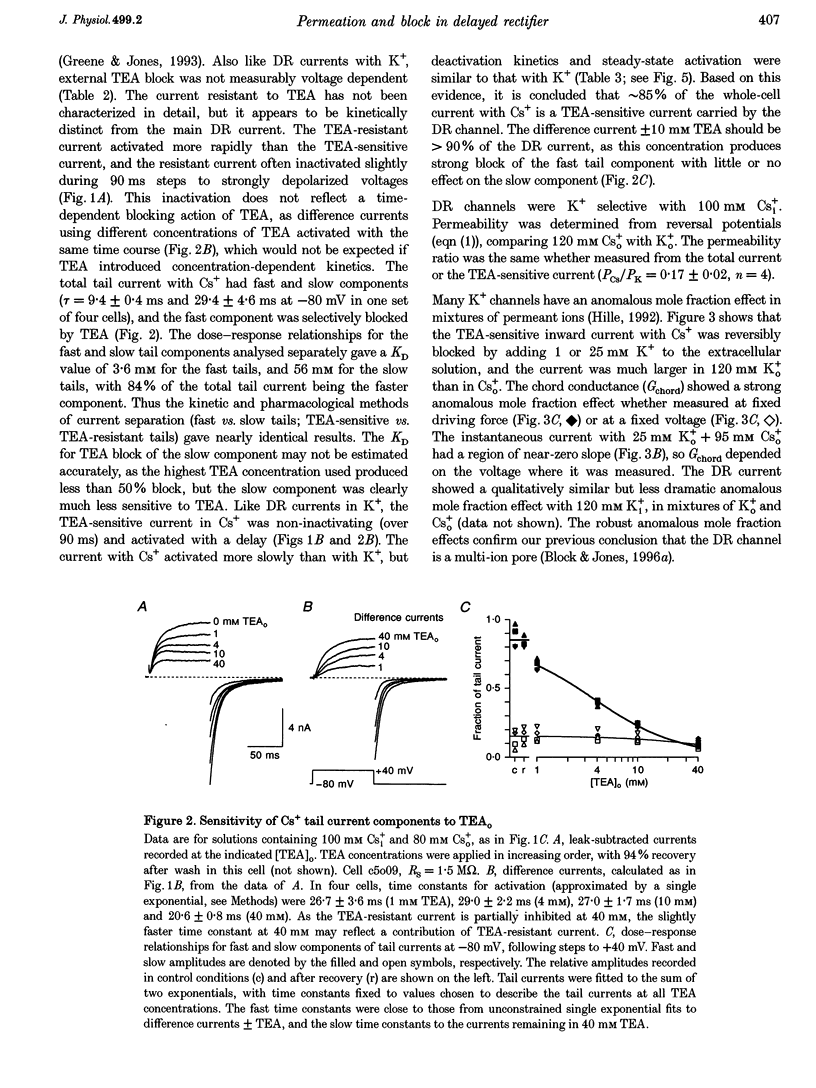
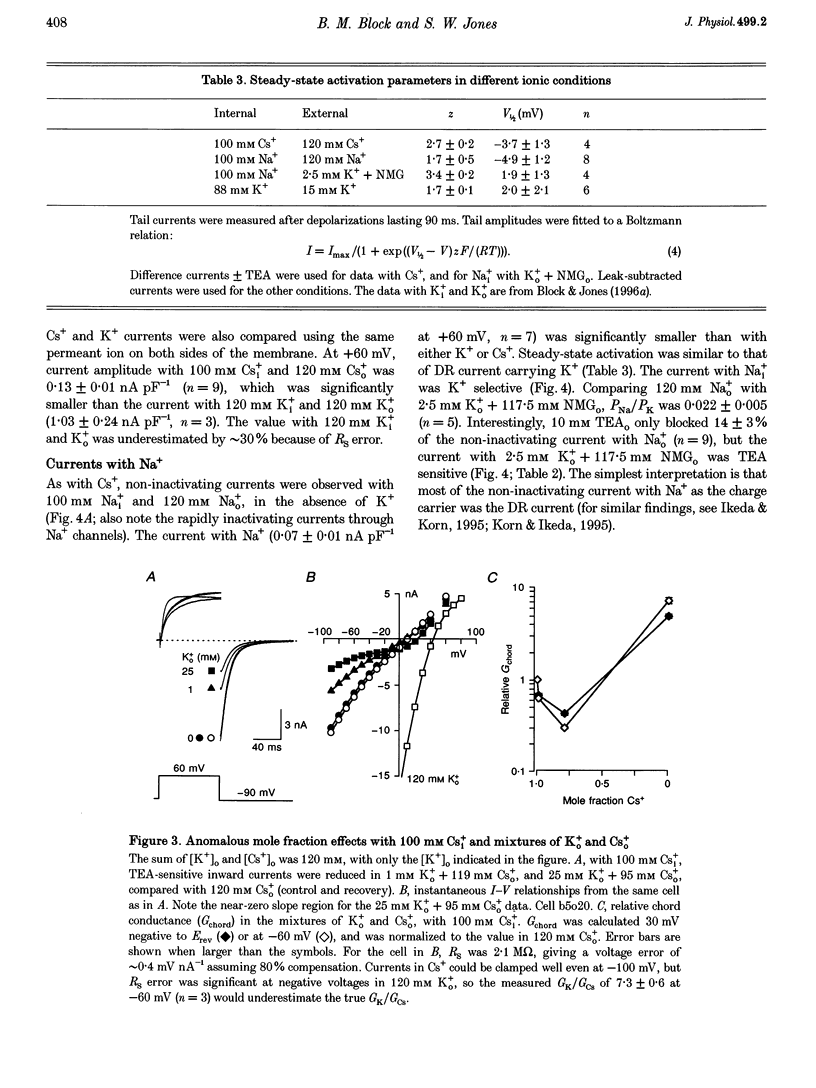
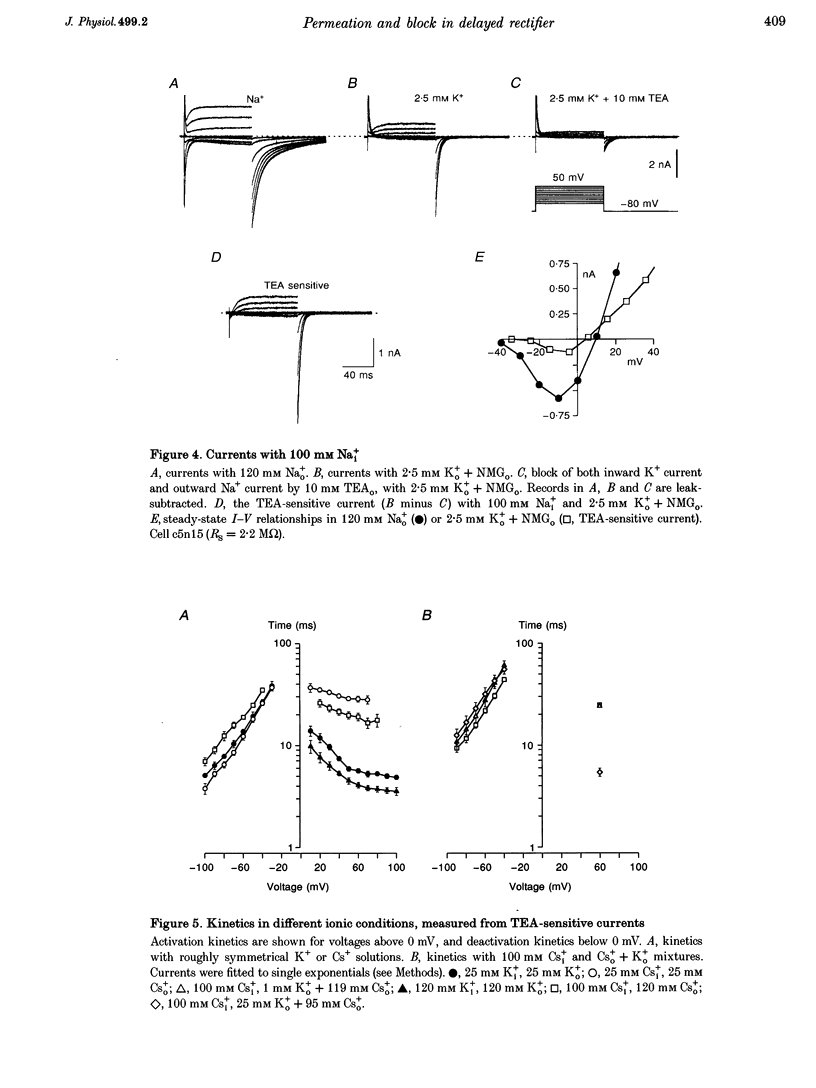
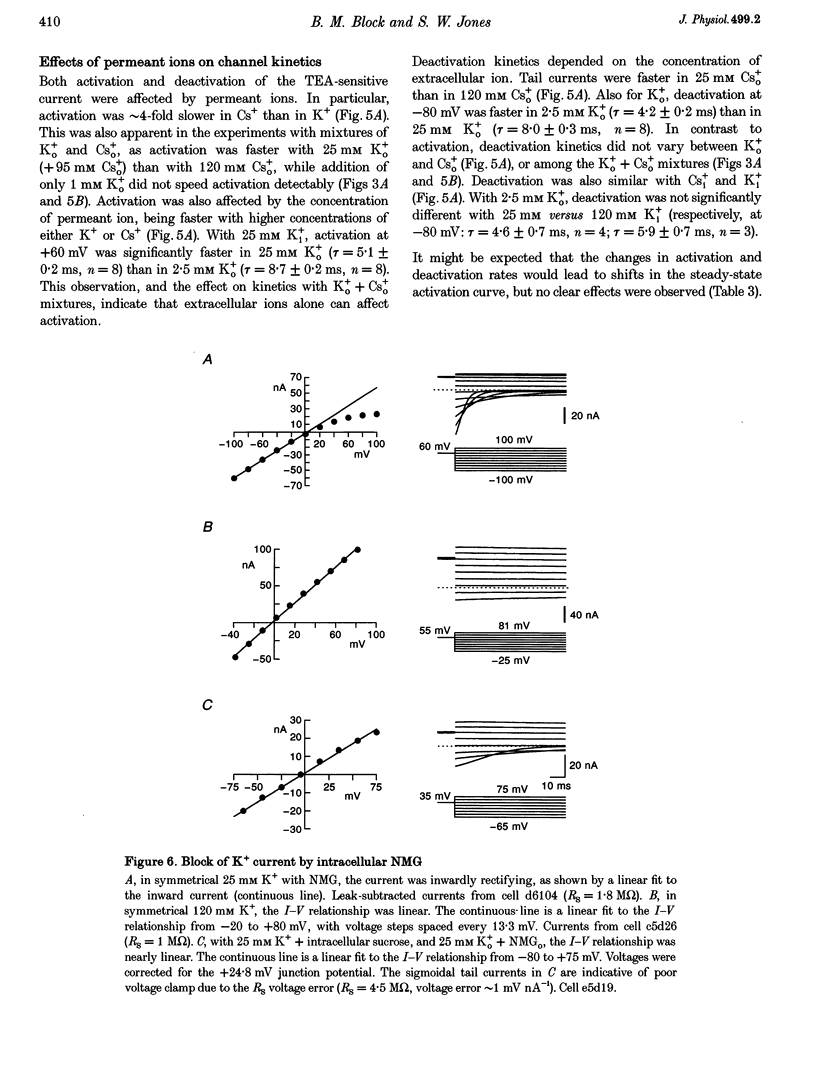
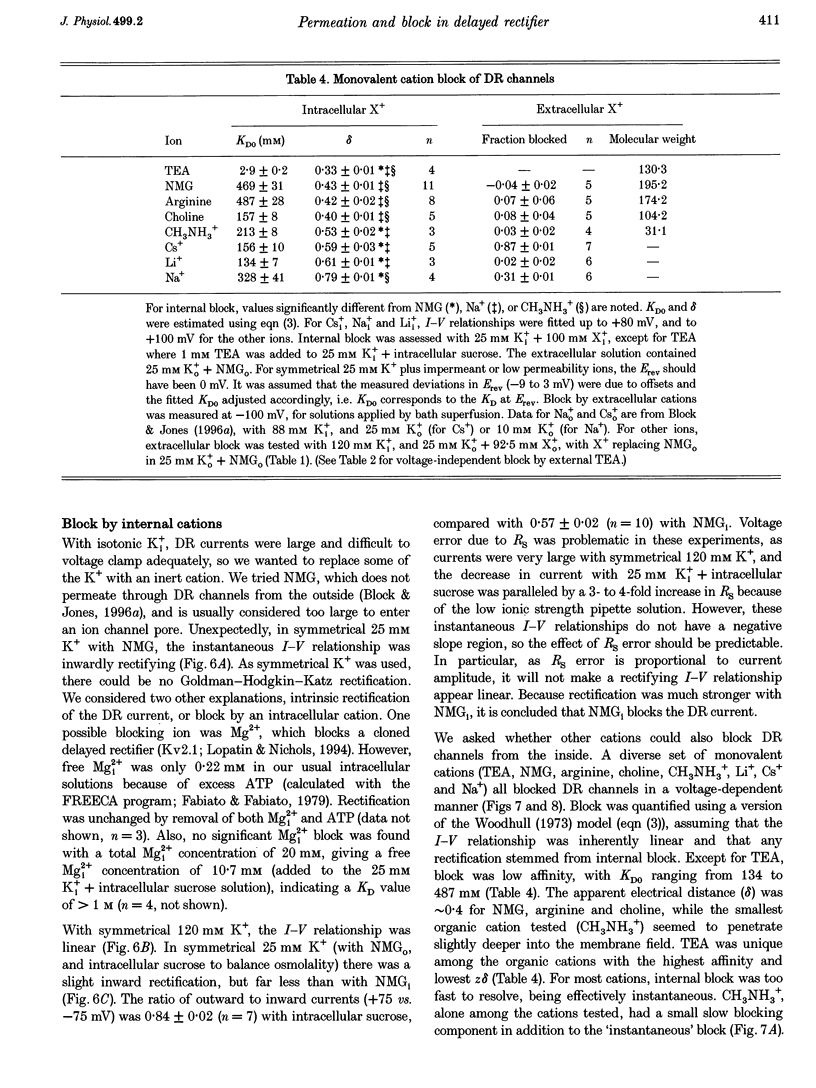
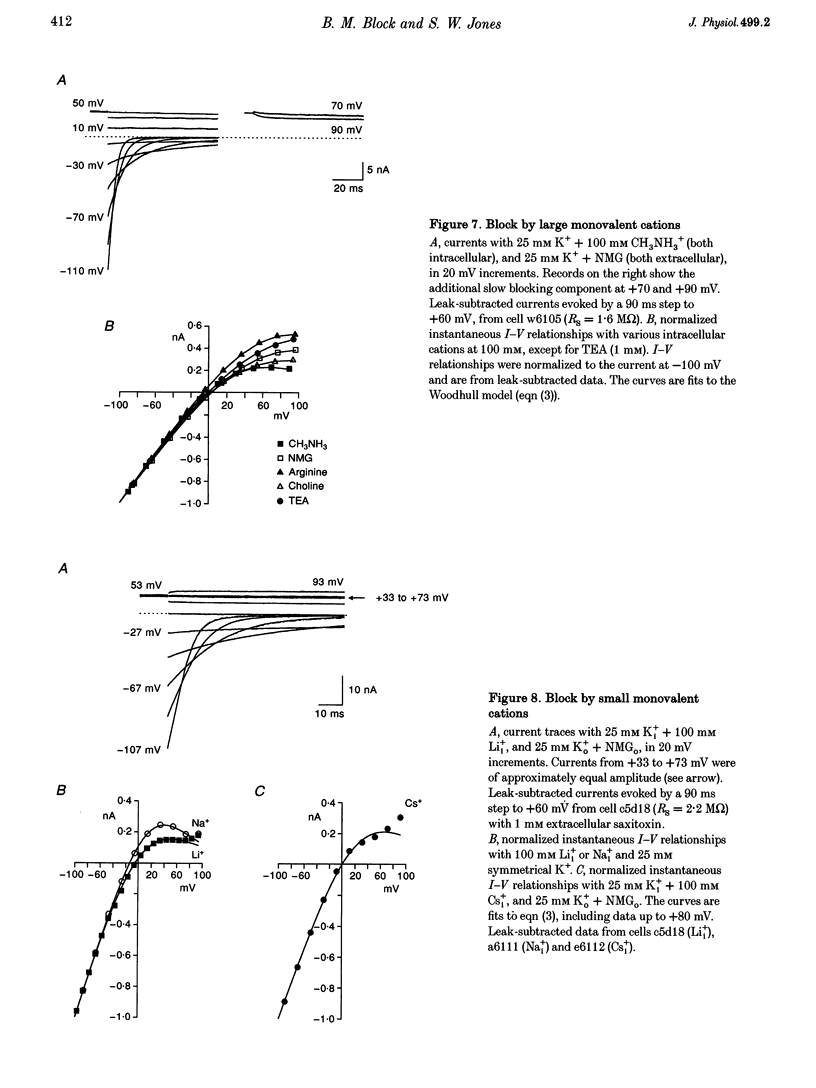
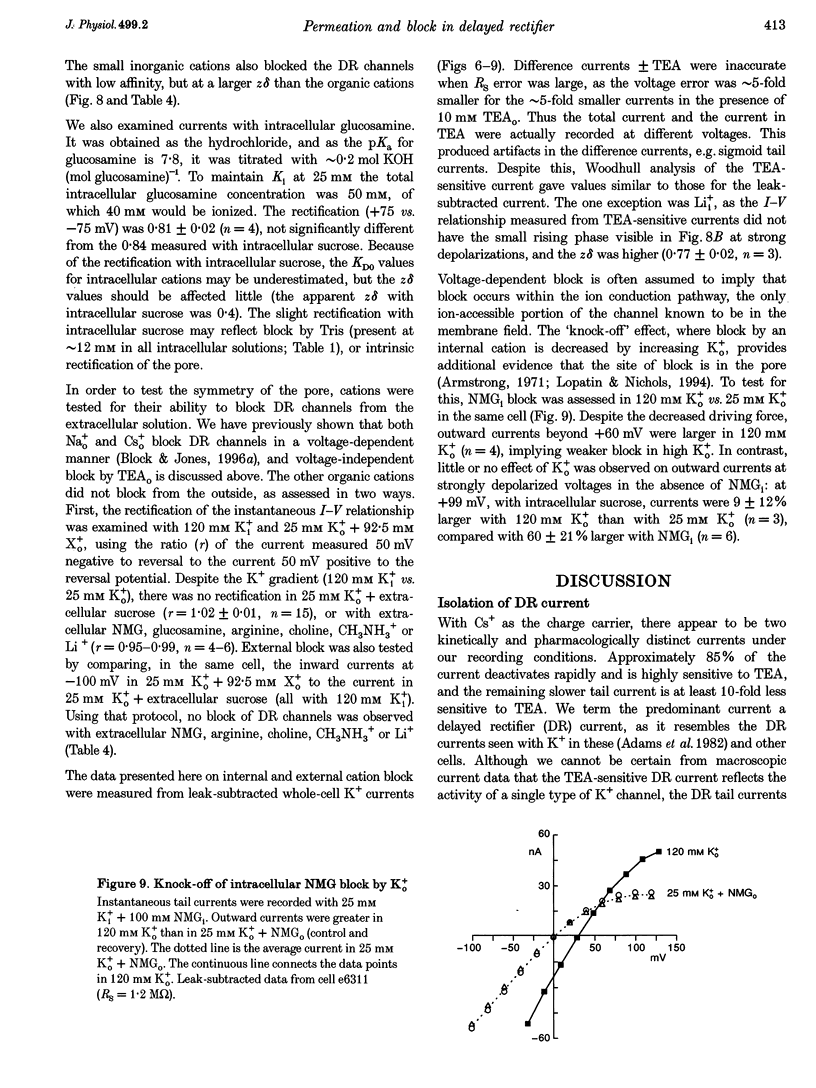
1. The delayed rectifier (DR) K+ channel pore was probed using different permeant and blocking ions applied intra- and extracellularly. Currents were recorded from bullfrog sympathetic neurons using whole-cell patch-clamp techniques. 2. With intra- and extracellular Cs+ (0 K+), there were large, tetraethylammonium (TEA)-sensitive currents. Adding K+ back to the extracellular solution revealed that the current with Cs+i was K+ selective (permeability ratio PCs/PK = 0.17 +/- 0.02, n = 4) and showed a strong anomalous mole fraction effect. 3. There were also large non-inactivating currents with Na+i and Na+o (0 K+). The current with Na+i was K+ selective (Na+o vs. K+o: PNa/PK = 0.022 +/- 0.005, n = 5), and was TEA sensitive with K+o but not with Na+o. 4. Permeant ions affected gating kinetics. DR currents activated faster in K+ than in Cs+, and activated faster with increasing concentrations of either K+ or Cs+. Deactivation was slowed by increased K+ or Cs+ concentration, with no difference between K+ and Cs+. 5. The pore was also characterized using intracellular blocking ions. A wide variety of monovalent cations (TEA, N-methyl-D-glucamine, arginine, choline, CH3NH3+, Li+, Cs+ and Na+) blocked DR channels from the inside in a voltage-dependent manner: KD at 0 mV was 2.9 mM for TEA and 134-487 mM for the others, at apparent electrical distances (delta) of 0.33-0.79. There was no detectable block by 10 mM Mgi2+. Apart from TEA, the organic cations did not block from the outside. 6. The permeability to Na+ in the absence of K+, and the strong anomalous mole fraction effects observed for Cs+o + K+o mixtures, suggest that DR channels select for K+ using ion-ion competition. The block by large intracellular cations shows that the pore is asymmetrical. The loss of high affinity TEAo block with Na+i and Na+o, and the effects of permeant ions on gating, suggest that channel conformation may be affected by ions in the pore.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Neyton J. Ion permeation through calcium channels. A one-site model. Ann N Y Acad Sci. 1991;635:18–25. doi: 10.1111/j.1749-6632.1991.tb36477.x. [DOI] [PubMed] [Google Scholar]
- Barry P. H., Lynch J. W. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991 Apr;121(2):101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972 Nov;60(5):588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block B. M., Jones S. W. Ion permeation and block of M-type and delayed rectifier potassium channels. Whole-cell recordings from bullfrog sympathetic neurons. J Gen Physiol. 1996 Apr;107(4):473–488. doi: 10.1085/jgp.107.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan M. J., Korn S. J. Permeation of Na+ through a delayed rectifier K+ channel in chick dorsal root ganglion neurons. J Gen Physiol. 1994 Oct;104(4):747–771. doi: 10.1085/jgp.104.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi X., Wolff D., Alvarez O., Latorre R. Mechanisms of Cs+ blockade in a Ca2+-activated K+ channel from smooth muscle. Biophys J. 1987 Nov;52(5):707–716. doi: 10.1016/S0006-3495(87)83265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. L., Mossman C., Aubé J., Yellen G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 1993 Mar;10(3):533–541. doi: 10.1016/0896-6273(93)90340-w. [DOI] [PubMed] [Google Scholar]
- Cukierman S., Yellen G., Miller C. The K+ channel of sarcoplasmic reticulum. A new look at Cs+ block. Biophys J. 1985 Sep;48(3):477–484. doi: 10.1016/S0006-3495(85)83803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo S. D., Yellen G. Ion effects on gating of the Ca(2+)-activated K+ channel correlate with occupancy of the pore. Biophys J. 1992 Mar;61(3):639–648. doi: 10.1016/S0006-3495(92)81869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- French R. J., Shoukimas J. J. An ion's view of the potassium channel. The structure of the permeation pathway as sensed by a variety of blocking ions. J Gen Physiol. 1985 May;85(5):669–698. doi: 10.1085/jgp.85.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R. J., Shoukimas J. J. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys J. 1981 May;34(2):271–291. doi: 10.1016/S0006-3495(81)84849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R. J., Wells J. B. Sodium ions as blocking agents and charge carriers in the potassium channel of the squid giant axon. J Gen Physiol. 1977 Dec;70(6):707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R., Korn S. J. Influence of permeating ions on potassium channel block by external tetraethylammonium. J Physiol. 1995 Jul 15;486(Pt 2):267–272. doi: 10.1113/jphysiol.1995.sp020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. Sodium currents in dissociated bull-frog sympathetic neurones. J Physiol. 1987 Aug;389:605–627. doi: 10.1113/jphysiol.1987.sp016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. Time course of receptor-channel coupling in frog sympathetic neurons. Biophys J. 1991 Aug;60(2):502–507. doi: 10.1016/S0006-3495(91)82077-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Ikeda S. R. Permeation selectivity by competition in a delayed rectifier potassium channel. Science. 1995 Jul 21;269(5222):410–412. doi: 10.1126/science.7618108. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Sejnowski T. J. Peptidergic and muscarinic excitation at amphibian sympathetic synapses. J Physiol. 1983 Aug;341:257–278. doi: 10.1113/jphysiol.1983.sp014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin A. N., Nichols C. G. Internal Na+ and Mg2+ blockade of DRK1 (Kv2.1) potassium channels expressed in Xenopus oocytes. Inward rectification of a delayed rectifier. J Gen Physiol. 1994 Feb;103(2):203–216. doi: 10.1085/jgp.103.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Swenson R. P., Jr External monovalent cations that impede the closing of K channels. J Gen Physiol. 1986 May;87(5):795–816. doi: 10.1085/jgp.87.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Neyton J., Pelleschi M. Multi-ion occupancy alters gating in high-conductance, Ca(2+)-activated K+ channels. J Gen Physiol. 1991 Apr;97(4):641–665. doi: 10.1085/jgp.97.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. S., DeCoursey T. E. Permeant ion effects on the gating kinetics of the type L potassium channel in mouse lymphocytes. J Gen Physiol. 1991 Jun;97(6):1251–1278. doi: 10.1085/jgp.97.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampe P., Begenisich T. Unidirectional K+ fluxes through recombinant Shaker potassium channels expressed in single Xenopus oocytes. J Gen Physiol. 1996 Apr;107(4):449–457. doi: 10.1085/jgp.107.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Jr, Armstrong C. M. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 1981 Jun 4;291(5814):427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Vandongen A. M., Drewe J. A., Joho R. H., Brown A. M., Kirsch G. E. Patterns of internal and external tetraethylammonium block in four homologous K+ channels. Mol Pharmacol. 1991 Aug;40(2):299–307. [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ellinor P. T., Sather W. A., Zhang J. F., Tsien R. W. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993 Nov 11;366(6451):158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Ikeda S. R. Anomalous permeation of Na+ through a putative K+ channel in rat superior cervical ganglion neurones. J Physiol. 1993 Aug;468:441–461. doi: 10.1113/jphysiol.1993.sp019781. [DOI] [PMC free article] [PubMed] [Google Scholar]


