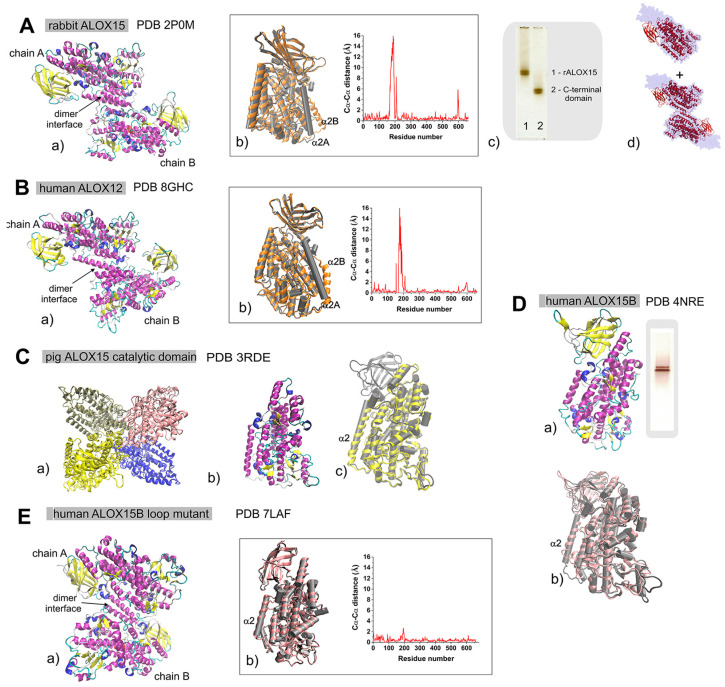
Figure 2.
Structures of different mammalian ALOX. (A) Structure of rabbit ALOX15 (15-LOX1) (PDB 2P0M). (a) Ribbon diagram of the dimer structure: α-helices (purple), 310-helices (blue), and β-sheets (yellow). (b) Overlay of crystal structures of monomer A (chain A, grey) and ligand-bound monomer B (chain B, orange). The Cα···Cα distances of monomers A and B versus residue number are plotted. (c) Native PAGE analysis of pure rabbit ALOX15 and its catalytic domain. (d) Overlay of the low-resolution structures [79] of pure native rabbit ALOX15 obtained in aqueous solutions (light blue) with the crystal structures of ALOX15 monomers and dimers. (B) Structure of human ALOX12 (platelet-type 12-LOX) (PDB 8HGC). (a) Ribbon diagram of the dimer structure: α-helices (purple), 310-helices (blue), and β-sheets (yellow). (b) Overlay of the crystal structures of monomer A (chain A, grey) and monomer B (chain B, orange). The Cα···Cα distances of monomers A and B versus residue number are plotted. (C) Structure of pig ALOX15 (leukocyte-type 12-LOX) catalytic domain (PDB 3RDE). (a) Ribbon diagram of the tetramer structure in the enzyme crystals. For clarity, different chains are labelled by different colors. (b) Folding of the catalytic domain of pig ALOX15: α-helices (purple), 310-helices (blue), and β-sheets (yellow). (c) Overlay of the crystal structures of rabbit ALOX15 (PDB 2P0M, monomer A, grey) and pig ALOX15 catalytic domain (PDB 3RDE, yellow). (D) Structure of human ALOX15B (15-LOX2) (PDB 4NRE) [12]. (a) Folding of human ALOX15B: α-helices (purple), 310-helices (blue), and β-sheets (yellow) and native PAGE of the pure recombinant enzyme. (b) Overlay of crystal structures of rabbit ALOX15 (PDB 2P0M, monomer A, grey) and human ALOX15B (PDB 4NRE, pink). (E) Structure of the human ALOX15B (15-LOX2) loop mutant dimer complex with ligand (PDB 7LAF) [13]. (a) Ribbon diagram of the dimer structure: α-helices (purple), 310-helices (blue), and β-sheets (yellow). (b) Overlay of the crystal structures of chain A (grey) and chain B (pink). The Cα···Cα distances versus residue number are plotted.