Abstract
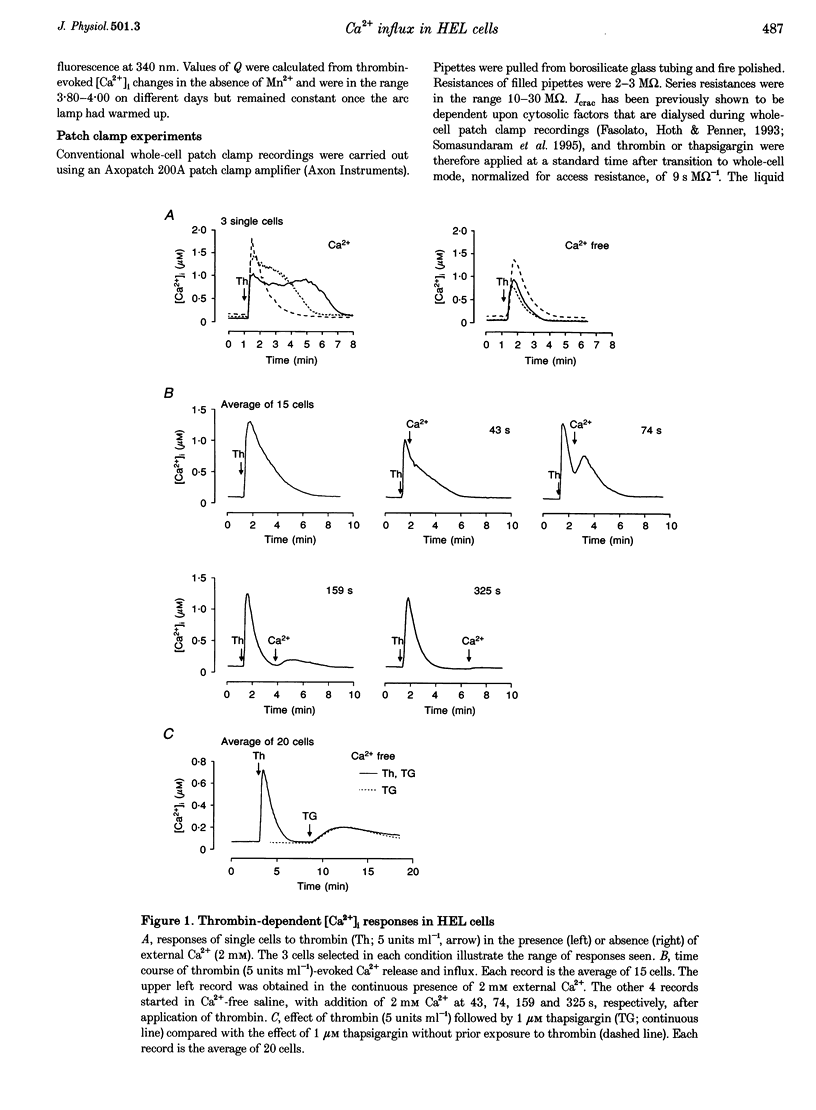
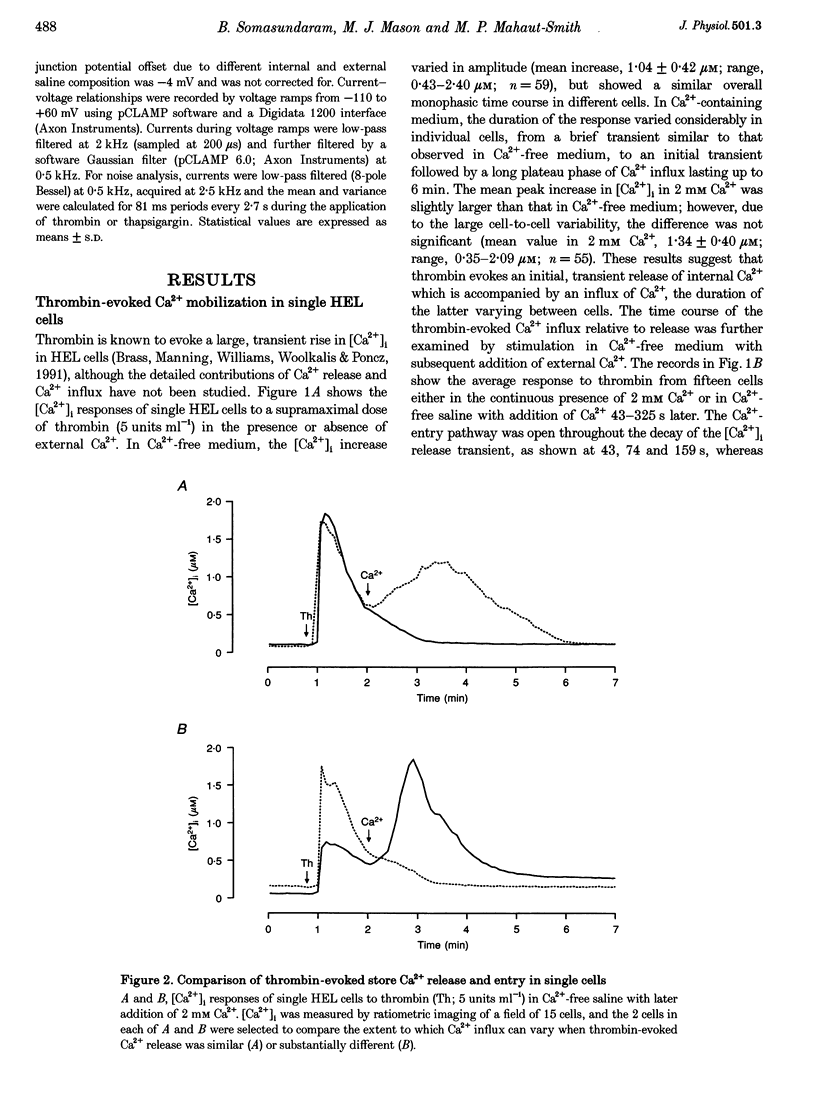
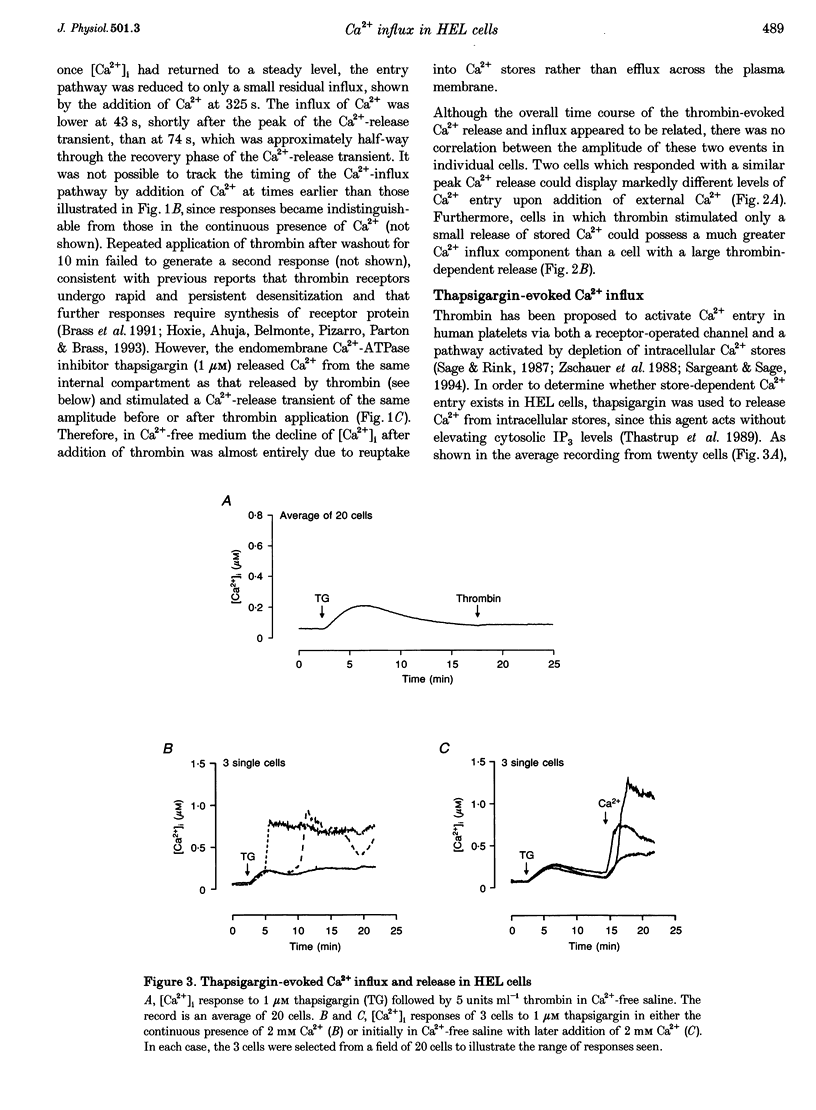
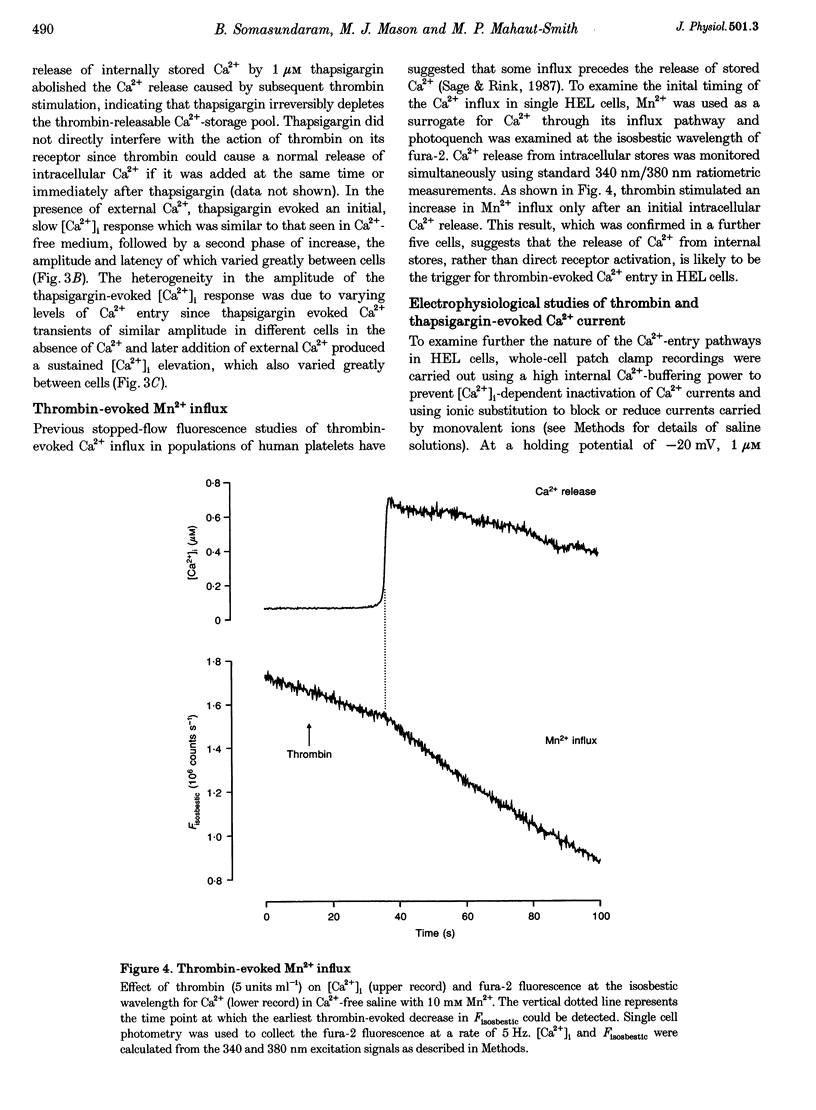
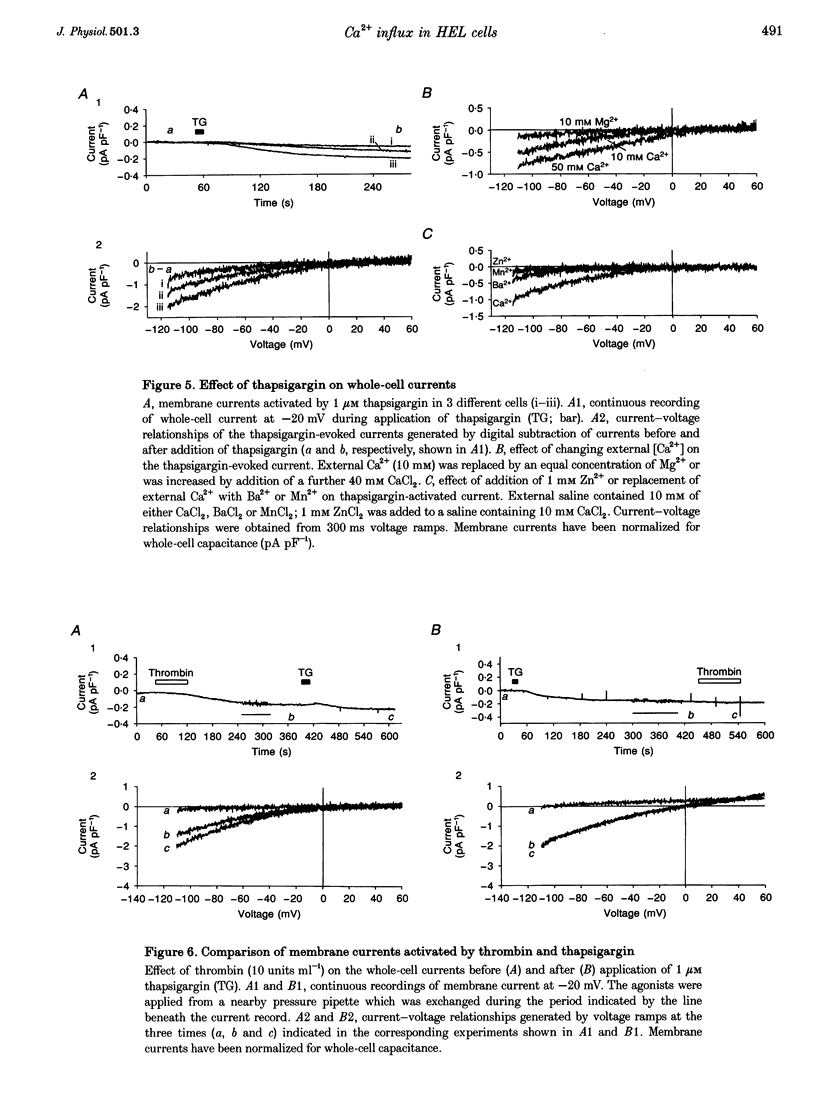
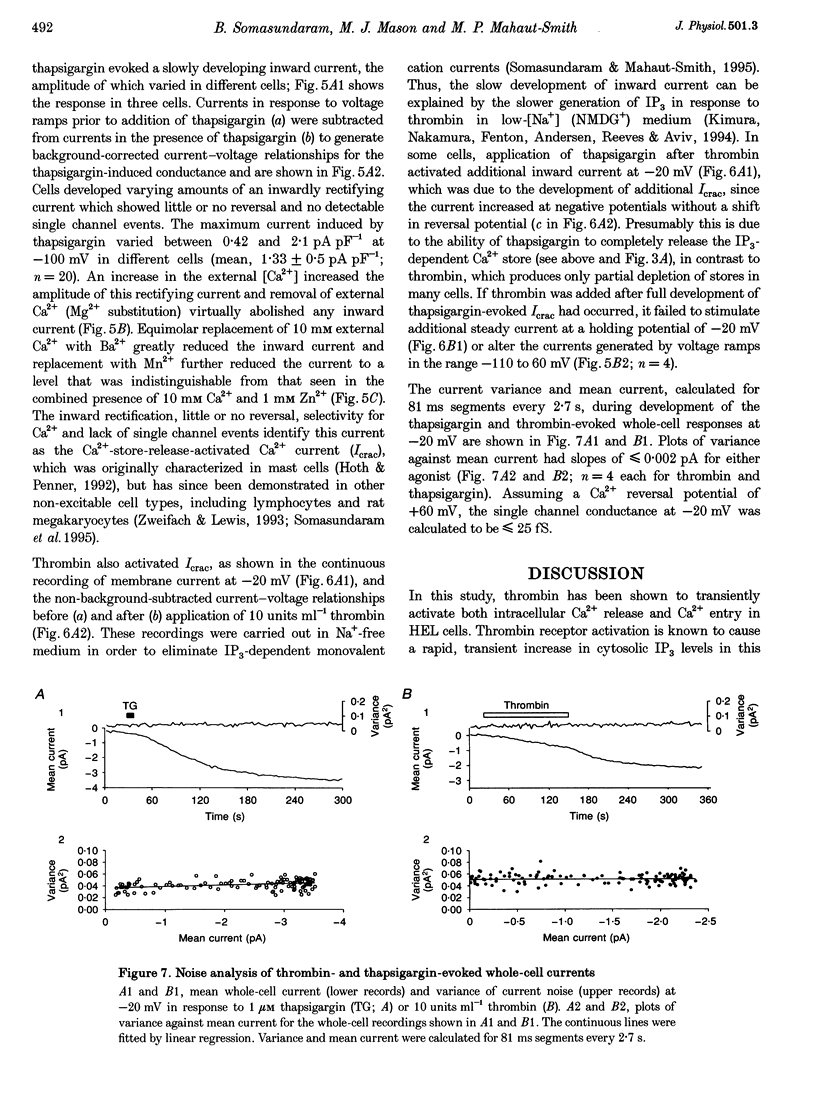
1. A combination of single cell fluorescence and patch clamp techniques were used to study the mechanisms underlying thrombin-evoked Ca2+ signals in human erythroleukaemia (HEL) cells, a leukaemic cell line of platelet-megakaryocyte lineage. 2. Thrombin caused a transient increase in intracellular Ca2+ ([Ca2+]i), consisting of both release of Ca2+ from intracellular stores and influx of extracellular Ca2+. Mn2+ quench studies indicated that the thrombin-evoked divalent cation-permeable pathway was activated during, but not prior to, release from internal stores. 3. Thapsigargin (1 microM) irreversibly released internal Ca2+ from the same store as that released by thrombin and continuously activated a Ca(2+)-influx mechanism. The amplitude of the thrombin- and thapsigargin-induced Ca2+ influx displayed a marked single cell heterogeneity which showed no correlation with the size of the store Ca2+ transient. 4. In whole-cell patch clamp recordings, both thrombin and thapsigargin evoked an inwardly rectifying Ca2+ current which developed with little or no increase in current noise, showed no reversal in the voltage range -110 to +60 mV and was blocked by 1 mM Zn2+. The apparent divalent cation permeability sequence of this pathway was Ca2+ > > Ba2+ > Mn2+, Mg2+. The thapsigargin-evoked current density at -100 mV varied between 0.42 and 2.1 pA pF-1 in different cells. Thrombin failed to activate additional Ca2+ current if it was added after the thapsigargin-induced inward current had fully developed. 5. These studies indicate that thrombin activates Ca2+ influx in HEL cells entirely via a Ca(2+)-store-release-activated Ca2+ current (Icrac) rather than via receptor-operated or second messenger-dependent Ca2+ channels. The level of expression of Icrac appears to be a major factor in determining the duration of the thrombin-evoked [Ca2+]i response and therefore represents a means by which cells can exert control over [Ca2+]i-dependent events.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Azula F. J., Alonso R., Marino A., Trueba M., Macarulla J. M. Ni2+ impairs thrombin-induced signal transduction by acting on the agonist and/or receptor in human platelets. Am J Physiol. 1993 Dec;265(6 Pt 1):C1681–C1688. doi: 10.1152/ajpcell.1993.265.6.C1681. [DOI] [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass L. F., Manning D. R., Williams A. G., Woolkalis M. J., Poncz M. Receptor and G protein-mediated responses to thrombin in HEL cells. J Biol Chem. 1991 Jan 15;266(2):958–965. [PubMed] [Google Scholar]
- Connolly A. J., Ishihara H., Kahn M. L., Farese R. V., Jr, Coughlin S. R. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996 Jun 6;381(6582):516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R. Molecular mechanisms of thrombin signaling. Semin Hematol. 1994 Oct;31(4):270–277. [PubMed] [Google Scholar]
- Fasolato C., Hoth M., Penner R. A GTP-dependent step in the activation mechanism of capacitative calcium influx. J Biol Chem. 1993 Oct 5;268(28):20737–20740. [PubMed] [Google Scholar]
- Fujimoto T., Fujimura K., Kuramoto A. Electrophysiological evidence that glycoprotein IIb-IIIa complex is involved in calcium channel activation on human platelet plasma membrane. J Biol Chem. 1991 Sep 5;266(25):16370–16375. [PubMed] [Google Scholar]
- Goodwin C. A., Wheeler-Jones C. P., Kakkar V. V., Deadman J. J., Authi K. S., Scully M. F. Thrombin receptor activating peptide does not stimulate platelet procoagulant activity. Biochem Biophys Res Commun. 1994 Jul 15;202(1):321–327. doi: 10.1006/bbrc.1994.1930. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hashimoto Y., Ogihara A., Nakanishi S., Matsuda Y., Kurokawa K., Nonomura Y. Two thrombin-activated Ca2+ channels in human platelets. J Biol Chem. 1992 Aug 25;267(24):17078–17081. [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Ahuja M., Belmonte E., Pizarro S., Parton R., Brass L. F. Internalization and recycling of activated thrombin receptors. J Biol Chem. 1993 Jun 25;268(18):13756–13763. [PubMed] [Google Scholar]
- Jenner S., Farndale R. W., Sage S. O. Wortmannin inhibits store-mediated calcium entry and protein tyrosine phosphorylation in human platelets. FEBS Lett. 1996 Mar 4;381(3):249–251. doi: 10.1016/0014-5793(96)00130-5. [DOI] [PubMed] [Google Scholar]
- Kimura M., Nakamura K., Fenton J. W., 2nd, Andersen T. T., Reeves J. P., Aviv A. Role of external Na+ and cytosolic pH in agonist-evoked cytosolic Ca2+ response in human platelets. Am J Physiol. 1994 Dec;267(6 Pt 1):C1543–C1552. doi: 10.1152/ajpcell.1994.267.6.C1543. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith M. P., Sage S. O., Rink T. J. Rapid ADP-evoked currents in human platelets recorded with the nystatin permeabilized patch technique. J Biol Chem. 1992 Feb 15;267(5):3060–3065. [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Michel M. C., Brass L. F., Williams A., Bokoch G. M., LaMorte V. J., Motulsky H. J. Alpha 2-adrenergic receptor stimulation mobilizes intracellular Ca2+ in human erythroleukemia cells. J Biol Chem. 1989 Mar 25;264(9):4986–4991. [PubMed] [Google Scholar]
- Nachman R. L., Leung L. L. Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J Clin Invest. 1982 Feb;69(2):263–269. doi: 10.1172/JCI110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M. Alteration of intracellular Fura-2 fluorescence by viscosity: a simple correction. Cell Calcium. 1990 Feb-Mar;11(2-3):85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- Preston S. F., Sha'afi R. I., Berlin R. D. Regulation of Ca2+ influx during mitosis: Ca2+ influx and depletion of intracellular Ca2+ stores are coupled in interphase but not mitosis. Cell Regul. 1991 Nov;2(11):915–925. doi: 10.1091/mbc.2.11.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Merritt J. E., Hallam T. J., Rink T. J. Receptor-mediated calcium entry in fura-2-loaded human platelets stimulated with ADP and thrombin. Dual-wavelengths studies with Mn2+. Biochem J. 1989 Mar 15;258(3):923–926. doi: 10.1042/bj2580923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 1987 Dec 5;262(34):16364–16369. [PubMed] [Google Scholar]
- Sargeant P., Sage S. O. Calcium signalling in platelets and other nonexcitable cells. Pharmacol Ther. 1994;64(3):395–443. doi: 10.1016/0163-7258(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Rajan L., Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity, and kinetics. J Biol Chem. 1989 Aug 5;264(22):12838–12848. [PubMed] [Google Scholar]
- Shi X. P., Yin K. C., Gardell S. J. Human erythroleukemic (HEL) cells express a platelet P2T-like ADP receptor. Thromb Res. 1995 Feb 1;77(3):235–247. doi: 10.1016/0049-3848(95)91611-n. [DOI] [PubMed] [Google Scholar]
- Siess W., Weber P. C., Lapetina E. G. Activation of phospholipase C is dissociated from arachidonate metabolism during platelet shape change induced by thrombin or platelet-activating factor. Epinephrine does not induce phospholipase C activation or platelet shape change. J Biol Chem. 1984 Jul 10;259(13):8286–8292. [PubMed] [Google Scholar]
- Somasundaram B., Mahaut-Smith M. P. A novel monovalent cation channel activated by inositol trisphosphate in the plasma membrane of rat megakaryocytes. J Biol Chem. 1995 Jul 14;270(28):16638–16644. doi: 10.1074/jbc.270.28.16638. [DOI] [PubMed] [Google Scholar]
- Somasundaram B., Norman J. C., Mahaut-Smith M. P. Primaquine, an inhibitor of vesicular transport, blocks the calcium-release-activated current in rat megakaryocytes. Biochem J. 1995 Aug 1;309(Pt 3):725–729. doi: 10.1042/bj3090725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabilio A., Rosa J. P., Testa U., Kieffer N., Nurden A. T., Del Canizo M. C., Breton-Gorius J., Vainchenker W. Expression of platelet membrane glycoproteins and alpha-granule proteins by a human erythroleukemia cell line (HEL). EMBO J. 1984 Feb;3(2):453–459. doi: 10.1002/j.1460-2075.1984.tb01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Harootunian A. T. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 1990 Feb-Mar;11(2-3):93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Zschauer A., van Breemen C., Bühler F. R., Nelson M. T. Calcium channels in thrombin-activated human platelet membrane. Nature. 1988 Aug 25;334(6184):703–705. doi: 10.1038/334703a0. [DOI] [PubMed] [Google Scholar]
- Zweifach A., Lewis R. S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A., Lewis R. S. Slow calcium-dependent inactivation of depletion-activated calcium current. Store-dependent and -independent mechanisms. J Biol Chem. 1995 Jun 16;270(24):14445–14451. doi: 10.1074/jbc.270.24.14445. [DOI] [PubMed] [Google Scholar]


