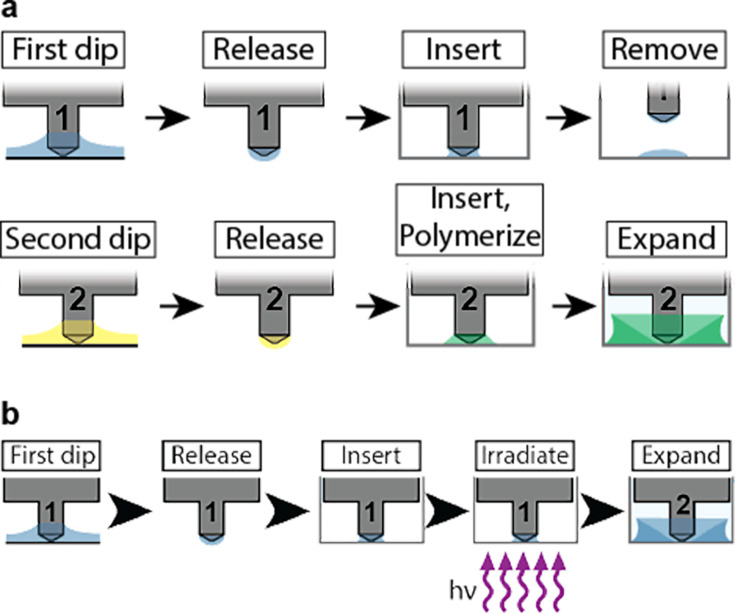
Figure 1. High-throughput expansion microscopy (HiExM) enables gel formation and expansion in a 96-well cell culture plate.
(a) Schematic representation of HiExM devices showing the key features highlighted in color. (b) Example devices used in 96-well cell culture plates. (c) Brightfield image of the conical post-tip shows the pattern of grooves that mediate fluid retention. (d) Fluid retention at the conical post-tip of the device. Silhouettes taken by an optical comparator of the profile of a single post suspended above a surface (left) and in contact with a surface (right) show a fluid droplet interacting with the device. Upon device insertion, the gel solution fills the space under the conical post tip, forming the toroid gel. (e–h) Schematic of HiExM gel deposition and expansion workflow. (e) The device is immersed in a shallow reservoir of gel solution. (f) Upon removal, the tip of each device post retains a small volume of gel solution. (g) Gel solution is deposited by the device into the well centers of the cell culture plate. Brightfield image (right) shows gel geometry and size prior to expansion. Note that gels deposited in HiExM cover ~1.1 mm2 of the well surface to accommodate the expanded gel, and do not include cells outside the gel footprint. (h) Polymerization and expansion are performed with the device in place. Brightfield image (right) shows gel geometry and size after expansion.