Simple Summary
Locusts are significant agricultural pests; therefore, the identification of novel control targets for their management is of immense importance. FoxO, a downstream target gene of cellular nutrient and growth factors, oxidative stress responses, and insulin signaling pathways, plays a pivotal role in the growth, development, and reproduction of insects. FoxO silencing resulted in significant changes in the expressions of genes associated with reproduction and the Hippo pathway and significantly reduced ovary development. These findings indicate that FoxO regulates reproduction in L. migratoria through the Hippo signaling pathway: when impaired, the reproductive capacity function declines. In addition, FoxO-mediated energy mobilization is involved in the regulation of egg production. Overall, these results highlight the potential of targeting FoxO as a novel molecular approach for controlling L. migratoria.
Keywords: Locusta migratoria, RNAi, FoxO, Hippo pathway, reproduction
Abstract
FoxO is a downstream target gene of cellular nutrient and growth factors, oxidative stress responses, and insulin signaling pathways. It play a crucial role in insect growth, development, and reproduction. Locusta migratoria is a significant agricultural pest; therefore, the identification of novel control targets for its management is of significant importance. After injecting dsRNA to interfere with FoxO expression, we observed changes in the reproduction-related gene expression and ovary development through RT-qPCR and morphological observation. Simultaneously, the trehalose and glycogen contents were measured following RNAi. The results demonstrate that interference with FoxO significantly downregulates key genes in the Hippo pathway and Notch gene expression. In terms of carbohydrate metabolism, the trehalose content decreases significantly while the glycogen content increases markedly after FoxO silencing. Additionally, FoxO silencing considerably inhibits reproductive-related gene expression, resulting in delayed ovarian development. These findings indicate that FoxO regulates L. migratoria reproduction through the Hippo signaling pathway: when impaired, the reproductive capacity function declines. In addition, FoxO-mediated energy mobilization is involved in the regulation of egg production. These results indicate that the RNAi of FoxO may be a useful control strategy against L. migratoria.
1. Introduction
Reproduction is a crucial factor that influences the adaptability of insects [1]. In terms of insect reproduction, the occurrence of yolk directly impacts their reproductive capacity [2]. Yolk occurrence primarily involves vitellogenin (Vg) production in the fat body, its release into the hemolymph, and its uptake by mature oocytes [3,4]. In Locusta migratoria, developing oocytes selectively incorporate Vg from outside the egg through endocytosis mediated by the vitellogenin receptor (VgR) [5]. Once inside the oocyte, Vg is stored as crystalline vitellin, serving as a nutritional reserve for future embryonic development [6,7]. At the oogenesis stage, the Notch pathway is involved in the spatial and temporal regulation of follicle cell differentiation and proliferation [8,9]. In L. migratoria, the increase in JH expression ensures high Notch abundance, consequently contributing to successful egg production [10].
In addition, the insulin signaling pathway in insects can influence their reproduction by regulating Vg protein synthesis [4,11]. As downstream target genes for cellular nutrients, growth factors, oxidative stress responses, and insulin signaling pathways (IIS), FoxO exerts both activating and inhibitory functions through transcriptional regulation mediated by interactions with regulators [12,13]. It binds to multiple target gene promoters and further modulates physiological activities such as growth, development, and reproduction [14,15]. In insects, FoxO functions as a transcriptional repressor that binds to the promoter region of Vg. Upon phosphorylation, it is expelled from the cell nucleus, thereby triggering Vg synthesis [16,17]. FoxO exerts an impact on reproduction in various insects, including Cyrtorhinus lividipennis, Tribolium castaneum, and Blattella germanica [18,19,20]. In B. germanica, FoxO RNAi in fed females caused substantially reduced Vg expression and arrested oocyte growth [21]. Similarly, FoxO knockdown caused reductions in the Vg mRNA levels in fed T. castaneum adult females [22].
The Hippo signaling pathway is a cascade reaction that governs organ size by regulating cell growth, proliferation, and apoptosis. Additionally, it plays a pivotal role in stem cell renewal and tissue regeneration [23,24]. Its core constituents comprise Hippo (Hpo), Warts (Wts), and Yorkie (Yki), as well as the scaffold protein Salvador (Sav) [25,26,27,28]. Moreover, the Hippo pathway exerts essential control over the Notch receptor levels in follicle cells. The disruption of this pathway results in the aberrant differentiation of follicle cells, thereby impacting oocyte polarity [29,30]. In Drosophila, the Hippo pathway plays a crucial role in regulating follicle cell differentiation and oocyte polarity formation during ovarian development, in conjunction with the Notch, EGFR, and JAK-STAT pathways [30,31]. Both the EGFR and Hippo signaling pathways are indispensable for maintaining germ cell populations [32].
L. migratoria is a significant agricultural pest due to its short reproductive cycle, high reproduction rate, migratory behavior, and tendency to aggregate [33,34]. Therefore, the identification and exploration of novel locust control targets is of immense practical significance. In this study, we investigated the interplay between FoxO and the Hippo signaling pathway and elucidated the role of FoxO in regulating reproduction in L. migratoria. Our findings highlight the potential of targeting FoxO as a novel molecular approach for controlling L. migratoria.
2. Materials and Methods
2.1. Insects for Testing
Eggs of L. migratoria were purchased from a locust farm in Huaibei, Anhui Province. Locust eggs (50 g) were placed in a box (10 cm × 15 cm × 20 cm) with a layer of wet sand (2–3 cm) and reared at 30 ± 2 °C and 80% RH (relative humidity), with a 16 h light–8 h dark photoperiod. After hatching, the locusts were fed a mixture of fresh wheat seedlings and wheat bran. Approximately 200–300 individuals in each cage were placed in an insect cage (50 cm × 50 cm × 50 cm) in an artificial climate chamber. The feeding and temperature conditions were the same as those described above.
2.2. Bioinformatic Analysis of LmFoxO
The LmFoxO protein sequence (accession number QJX15634.1) was retrieved from GeneBank. The cDNA sequence of the FoxO gene was obtained from the locust transcriptomic database and was identified from genomic data on L. migratoria [35]. The ExPASy Proteomics website (http://web.expasy.org/protparam/ (accessed on 1 July 2021)) was used to predict the molecular mass and isoelectric point of LmFoxO. The SMART tool (http://smart.embl.de/ (accessed on 1 July 2021)) was used to predict the conserved structural domains of the FoxO protein. The BLAST search developed by the NCBI compared the homology of locusts with other species, selected the top 10 sequences with the highest identity, and used the multiple sequence results of MEGA 11 to build the evolutionary tree.
2.3. RNA Extraction and RT-qPCR
Total RNA was extracted using the Trizol reagent (TaKaRa, Dalian, China). The RNA concentration was determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Reverse transcription (RT) reactions were carried out using the PrimeScript RT Reagent Kit (Takara, Dalian, China). The cDNA was diluted 10 times for the subsequent general polymerase chain reaction (PCR), reverse transcription quantitative PCR (RT-qPCR), and dsRNA synthesis studies.
RT-qPCR was performed using a Bio-Rad Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). All RT-PCR primers were designed using Primer 5.0 software (Table 1). Lmβ-actin was used as the internal reference gene. The gene expressions of FoxO, Hpo, Sav, Yki, Met, and Vg were detected via real-time fluorescence quantitative PCR using 10.0 μL of the PCR reaction system, 5 μL of SYBR Premix Ex Taq (Takara, Japan), 0.4 μL of forward primer, 0.4 μL of reverse primer, 1 μL of template cDNA, and 3.2 μL of RNase-free ddH2O. The reaction procedure included an initial pre-denaturation at 95 °C for 3 min, followed by 32 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 10 min. The relative expressions of the target genes were calculated using the 2−△△CT method [36].
Table 1.
Primers for PCR.
| Primer Name | F-Primer Sequence [5′–3′] | R-Primer Sequence [5′–3′] | Method |
|---|---|---|---|
| FoxO1 | AGATGGACCCGTCGTTCGAG | GGCTGAAGTCTGAAGTTGAAGTC | cDNA Clones |
| FoxO2 | CTGGACGTGGTGGTGAAGCA | CGTGCTTGATCACCTCGTCC | |
| FoxO3 | GCCAAGAAGAACACCAGCC | CGTCTCGATGTTGAGGTTGAGG | |
| GFP | AAGGGCGAGGAGCTGTTCACCG | CAGCAGGACCATGTGATCGCGC | |
| FoxO | GAACTCGATCCGGCATAACC | CGCCTCCACCTTCTTCTTG | RT-qPCR |
| VgA | CCCACAAGAAGCACAGAACG | TTGGTCGCCATCAACAGAAG | |
| VgB | GCACTTAGCAGCATTAAGACCC | GGCAACGATAGATGGATAGGAC | |
| VgR1 | ATAAAGGTCTACCATCCAGCCC | GACAGGCACAGGTGTAGGAGTT | |
| VgR2 | GGCAAAAGGGATCACTCGA | GCCACCATCAGCCCAAAAT | |
| Met | GCGGTCACCTCTTGTCAATAAT | CACTTTCTGATGCTGCCCTAA | |
| Hpo | GCTGAAAACATAAAGGGAGG | CTGGAATGGATTCGGAGG | |
| Sav | CTGCTTTGGTTCCTTCAGT | GTTGGTAGCCCTTCTTTCTC | |
| Yki | AAGCCCCTGCTCGTATTTAT | TCTATCCGCACCACCAAGTT | |
| Notch | CGGAAACCGAGTGTCAAG | CGGGCTGGGAATGCTA | |
| dsFoxO | TAATACGACTCACTATAGGGAGATGGACCCGTCGTTCGAG | TAATACGACTCACTATAGGGGGCTGAAGTCTGAAGTTGAAGTC | dsRNA Synthesis |
| dsGFP | TAATACGACTCACTATAGGGAAGGGCGAGGAGCTGTTCACCG | TAATACGACTCACTATAGGGCAGCAGGACCATGTGATCGCGC |
2.4. Tissue Expression Analysis of FoxO
To investigate the tissue-specific expression pattern of FoxO, five tissues were dissected from adult locusts (12 h post-adult eclosion): ovary, fat body, integument, midgut, and brain tissues. All samples were collected with three biological replicates, with five locusts per sample. The samples were snap-frozen in liquid nitrogen and stored at −80 °C for the subsequent total RNA extraction. The tissue-specific expression pattern of FoxO was analyzed using RT-qPCR.
2.5. RNAi-Mediated FoxO Silencing
To further investigate the function of FoxO, we employed RNA interference (RNAi) to knock down the expressions of target genes, with the green fluorescent protein (GFP) gene serving as a negative control. Due to the high GC content in the FoxO genome, nested PCR was chosen for its amplification. The specific primers used for the FoxO PCR amplification and dsRNA synthesis were designed using Primer 5.0 software (Table 1). The thermal profile for the nested PCR consisted of an initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s and annealing at 55 °C for 30 s. The dsFoxO and dsGFP were synthesized in vitro using the T7RiboMAX Express RNAi System (Promega Corporation, Madison, WI, USA), and they were purified following Tenlen’s method, described previously [37]. The synthesized dsFoxO and dsGFP were dissolved in ddH2O, and the final concentration was adjusted to 2 μg/μL. Approximately 20 μg (10 μL) of dsFoxO was injected into the abdomen between the second and third abdominal segments of each female locust in the early eclosion phase. All locusts treated with dsRNA were maintained under identical conditions, as described above, for the subsequent analysis. Samples were collected 5 days after injection for further analyses. The other parts of the locusts were bred until the insects died, and the weight of each pod was weighed and recorded. At the same time, the pod was carefully opened with a writing brush, and the number of eggs in each pod was counted.
2.6. Glycogen and Trehalose Determination
For the glycogen and trehalose content measurements, hemolymph was collected 5 days after injection for subsequent analysis. Each group included three biological replicates of five locusts. The samples were then centrifuged at 4 °C for 20 min at 3500 rpm to remove the hemocytes. Subsequently, 5 µL of hemolymph was mixed with PBS (32 µL) and 10% trichloroacetic acid (148 µL). The mixture was then centrifuged at 4 °C for 2 min at 10,000 rpm as the test sample.
The glucose standard curve was prepared with the glucose standard solution and the standard dilution with concentrations of 0 mg/L, 0.02 mg/L, 0.04 mg/L, 0.06 mg/L, 0.08 mg/L, and 0.1 mg/L. An amount of 30 µL of the sample was tested, the standard solution was taken, and 600 µL of the developer was added to a 90 °C water bath for 10 min, followed by an ice bath for 3 min. After mixing, the reaction mixture was added to the enzyme label plate, and the absorbance (A0) was determined at a wavelength of 620 nm.
The trehalose standard was diluted on a concentration gradient with preparations of 0.8 mM, 0.4 mM, 0.2 mM, 0.1 mM, and 0.05 mM as the standard curve test samples. An amount of 30 μL of the test samples or standard samples was added to a 1.5 mL Eppendorf (EP) tube, and 30 μL of 1% H2SO4 was added, followed by a 90 °C water bath for 10 min and an ice bath for 3 min. An amount of 30 μL of 30% KOH was added, followed by a 90 °C water bath for 10 min and an ice bath for 3 min. An amount of 600 μL of the developer (600 μL of 0.02 g of anthrone in 100 mL 80% H2SO4) was added, followed by a 90 °C water bath for 10 min and an ice bath for 3 min. After mixing, the reaction mixture was added to the enzyme label plate, and the absorbance was determined at a wavelength of 630 nm.
2.7. Data Statistics and Analysis
Data are expressed as means ± standard errors (SEs) and were evaluated for their normality and homogeneity of variance. Statistical analysis was performed using SPSS 26.0 software. One-way analysis of variance (ANOVA) followed by Tukey’s multiple range test was used to compare the differences between the treatment and control groups, and Student’s t-tests were used for the independent samples. All experiments were performed in triplicate with three biological replicates and at least three technical replicates.
3. Results
3.1. Bioinformatics Analysis of FoxO
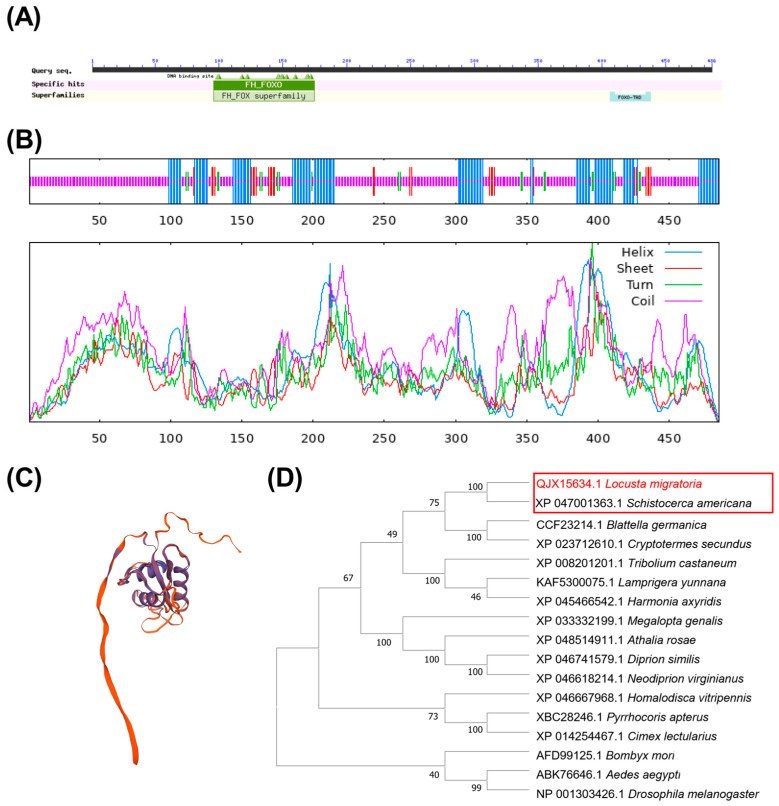
The cDNA sequence of LmFoxO (GenBank accession number QJX15634.1) was identified based on the transcriptome data. The predicted protein has a calculated molecular mass (MM) of approximately 52,186 and an isoelectric point (pI) of 9.30, as determined using the ExPASy Proteomics website. The amino acid sequence of FoxO consists of an FH domain spanning residues 95–175 and a FoxO-TAD domain spanning residues 406–438 (Figure 1A). The secondary structure analysis revealed that the FoxO protein comprises α-helices, extended chains, β-turns, and random coils (Figure 1B), with random coils constituting the largest proportion at 63.71%, which is consistent with the predicted tertiary structure (Figure 1C).
Figure 1.
Bioinformatics analysis of FoxO in L. migratoria. (A) Prediction of conserved domains in LmFoxO proteins, which contain two functional domains: the FH and FoxO-TAD structure domains. (B) Secondary structure of LmFoxO. (C) Tertiary structure of LmFoxO. (D) Evolutionary tree analysis of LmFoxO using the neighbor-joining method with insect FoxO protein sequences from S. americana, B. germanica, C. secundus, H. vitripennis, T. castaneum, H. axyridis, C. lectularius, L. yunnana, M. genalis, P. apterus, A. rosae, D. similis, N. virginianus, D. melanogaster, B. mori, and A. aegypti.
The similarity of locusts with other species was assessed using a BLAST search on the NCBI website, and the top 10 sequences with the highest identity were selected. The multiple sequence results obtained from MEGA 11 were utilized to construct the evolutionary tree. A significant level of homology was revealed in the amino acid sequence of FoxO between L. migratoria and Schistocerca americana (XP_047001363.1) (Figure 1D).
3.2. Tissue-Specific Expressions of FoxO and Key Hippo-Related Genes in L. migratoria
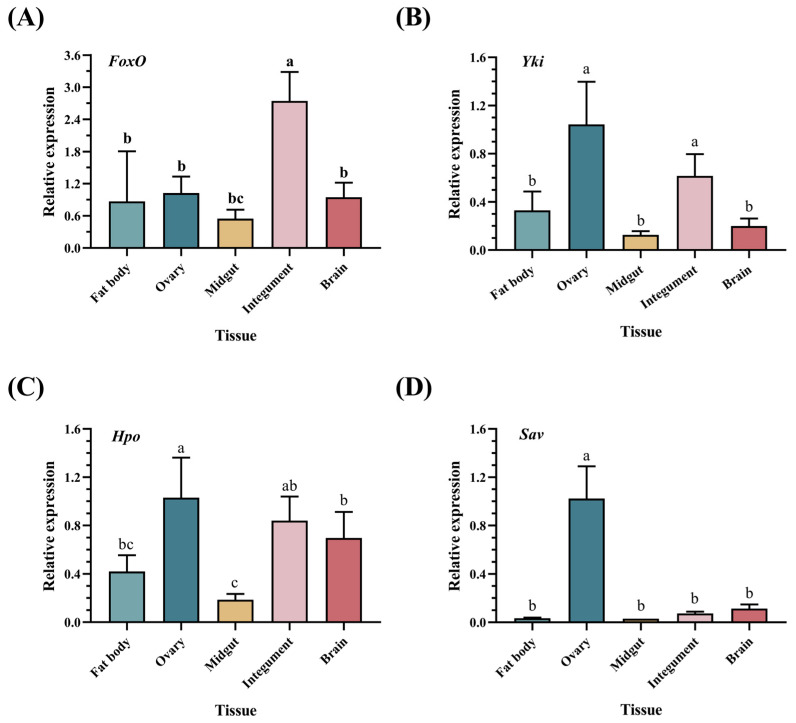
To investigate the tissue-specific expression patterns of FoxO and the key genes of the Hippo pathway, we performed RT-qPCR to detect the transcript levels in five tissues from female locusts. FoxO expression was detected in all five tissues, with predominant expression in the integument and relatively high expression in the ovary (Figure 2A). Yki exhibited prominent expression in the ovary and relatively high expression in the integument (Figure 2B). Hpo showed relatively higher expression levels in the ovary compared to the integument, and the lowest expression levels were observed in the midgut (Figure 2C). Sav displayed significantly higher expression levels in the ovary compared to the other tissues (Figure 2D). These findings suggest that LmFoxO and the Hippo pathway may play a role in the reproductive processes of L. migratoria.
Figure 2.
Relative expression of FoxO in different tissues. The tissue-specific expression patterns of FoxO and Hippo-related genes in L. migratoria, including (A) FoxO, (B) Yki, (C) Hpo, and (D) Sav in the fat bodies, ovaries, midguts, integuments, and brains of female adults within 12 h post-eclosion. The values are presented as means ± SEs (n = 3). Different letters indicate significant differences among the tissues (p < 0.05) based on one-way ANOVA. Three biological replicates were established for each developmental stage, with no fewer than five test worms.
3.3. Effects of dsFoxO on Expressions of FoxO and Key Hippo-Related Genes
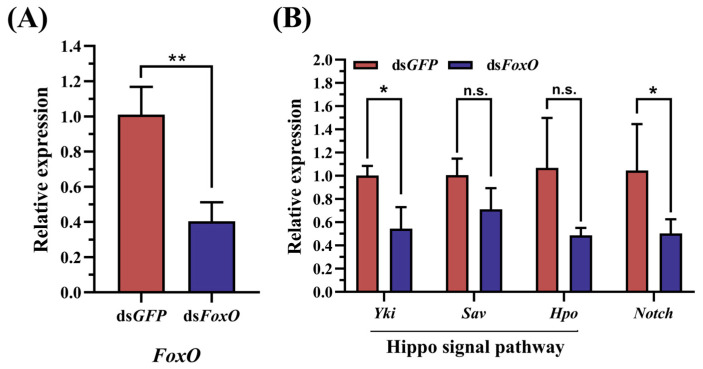
FoxO RNAi resulted in a significant 59.75% reduction in the transcript abundance of FoxO in the fat body of adult females at 5 days after the treatment (Figure 3A). To investigate the impact of LmFoxO interference on the key genes involved in the Hippo pathway, we assessed the expression levels of three crucial genes using RT-qPCR. The data revealed that the knockdown of LmFoxO effectively downregulated the Yki transcript levels, as well as reduced the expressions of the Sav and Hpo genes (Figure 3B), indicating that dsFoxO influenced the Hippo pathway and inhibited related gene expressions.
Figure 3.
Effect of RNAi on the relative expressions of FoxO and Hippo-related genes in L. migratoria. (A) Changes in relative expressions of FoxO genes following RNAi treatment. Impact of FoxO RNAi injection on the expressions of (B) Hippo-related genes and Notch. The control group received an equal injection volume of dsGFP. Values are presented as means ± standard errors (SEs). * Denotes a significant difference between the two groups using Student’s t-test (* p < 0.05 and ** p < 0.01), with three biological replicates consisting of no less than five test insects per treatment.
3.4. Effects of FoxO Silencing on L. migratoria Reproduction
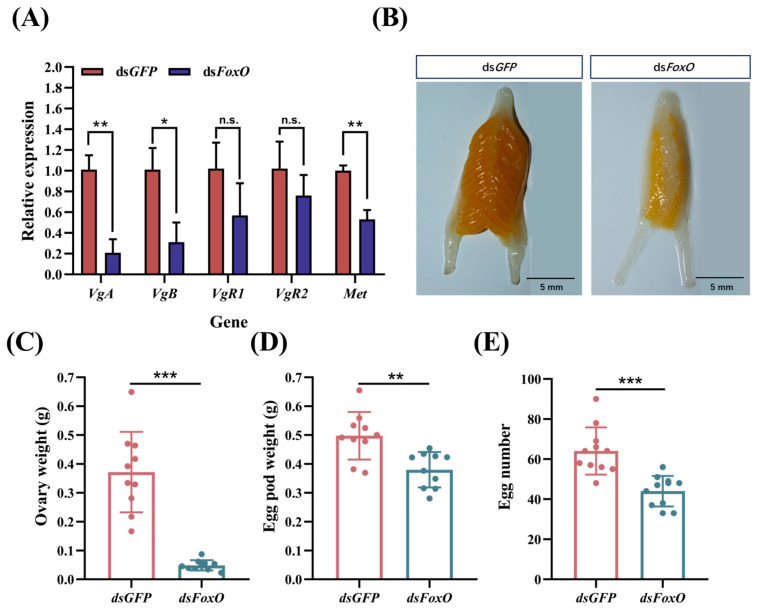
To investigate the impact of FoxO silencing on locust reproduction, we examined the expression levels of the reproduction-related genes as well as the ovarian development. Our findings revealed a significant downregulation in the mRNA levels of VgA, VgB, and Met in the fat body (Figure 4A), indicating the inhibition of vitellogenin synthesis. The expression levels of VgR1 and VgR2 in the ovary were also observed to be downregulated, although there was no significant difference. Furthermore, there was a notable reduction in ovarian weight and severe atrophy in ovarian development (Figure 4B,C). Additionally, we observed substantial decreases in both the egg pod weight and number following the dsFoxO injection (Figure 4D,E). These results underscore the profound impact of FoxO silencing on L. migratoria’s reproductive capabilities.
Figure 4.
Effects of FoxO silencing on reproduction in L. migratoria. (A) Impact of dsFoxO injection on the expressions of reproduction-related genes, including VgA, VgB, VgR1, VgR2, and Met. The values are presented as means ± SEs. Changes in the (B) ovarian morphology, (C) ovary weight, (D) egg pod weight, and (E) egg number after FoxO silencing were assessed. Statistical significance was determined using Student’s t-test (* p < 0.05, ** p < 0.01, and *** p < 0.001). Each treatment consisted of three biological replicates with no less than five test insects per replicate. The scale bar represents 5 mm.
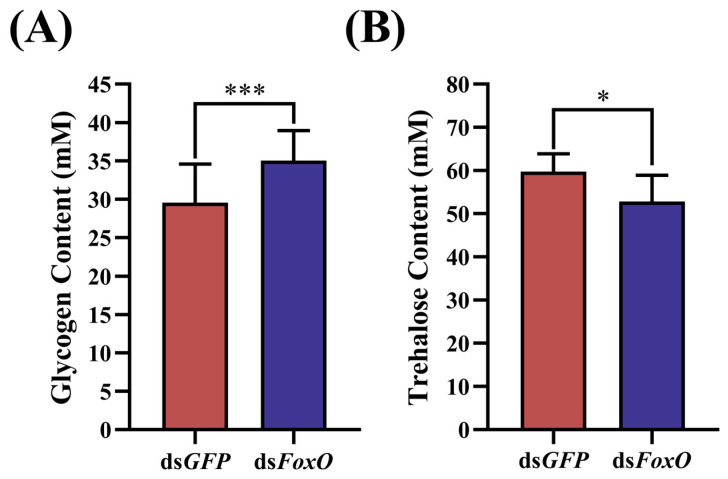
3.5. Effects of FoxO Silencing on Glycogen and Trehalose in L. migratoria
The active reproduction of insects is closely intertwined with their metabolism. Therefore, we aimed to explore whether FoxO contributes to the reproduction–metabolism balance in locusts. We measured the trehalose and glycogen levels of females under different experimental conditions. Upon dsFoxO injection, the locusts exhibited significantly increased glycogen contents but significantly decreased trehalose contents, indicating a regulatory role for FoxO (Figure 5).
Figure 5.
Effects of FoxO silencing on (A) glycogen and (B) trehalose in L. migratoria. The control group was injected with dsGFP. Values are presented as means ± SEs. * Denotes a significant difference between the two groups using Student’s t-test (* p < 0.05 and *** p < 0.001), with three biological replicates consisting of no less than five test insects per treatment.
4. Discussion
The Forkhead box (Fox) protein family, consisting of 19 subfamilies, is a widely distributed transcription factor family in animals that is characterized by a conserved DNA-binding domain (the Forkhead-box or Fox) [38,39]. Among these subfamilies, FoxO has been extensively studied and exhibits a highly conserved structure and function across species [14,40,41]. In this study, we identified LmFoxO and found that the amino acid sequence of FoxO consists of an FH domain and a FoxO-TAD domain (Figure 1A), which demonstrates the conservation of FoxO. The multiple sequence results obtained from MEGA 5.1 were utilized to construct the evolutionary tree. The multiple sequence alignment revealed a significant level of homology in the amino acid sequence of FoxO between L. migratoria and Schistocerca americana (XP_047001363.1) (Figure 1D).
Multiple studies have demonstrated that the Hippo signaling pathway serves as a primary target through which FoxO governs cellular homeostasis and lifespan regulation [26,42,43,44]. Additionally, gene ontology analysis has revealed the enrichment of differentially expressed FoxO target genes in aging fat bodies within the Hippo signaling pathway [45]. Previous studies employing ChIP-Seq technology have confirmed the Hippo pathway as a major target of FoxO in wild-type fruit flies [45]. Regulators of the Hippo pathway are among the FOXO-dependent upregulated genes [46] (Figure S1). Alternatively, via the STRING database, we predicted an interaction between FOXO proteins and the key proteins of the Hippo pathway in Drosophila (Figure S2; Table S1). These aforementioned investigations provide a theoretical foundation for exploring the relationship between FoxO and the Hippo signaling pathway, as well as their joint mechanisms that regulate insect reproduction. In female locusts with disrupted FoxO function, there was a significant reduction in the expressions of the key genes Yki, Hpo, and Sav (Figure 3). These findings confirm that the Hippo pathway is targeted by FoxO in L. migratoria.
In this study, we initially assessed the expression profiles of both FoxO and the key genes involved in the Hippo pathway. We observed the widespread expression of FoxO across various tissues in the female locusts, with predominant expression in the integument tissue and relatively high expression levels in the ovaries (Figure 2A), suggesting the potential involvement of FoxO in diverse biological processes, including reproduction. We selected female adult locusts that had undergone molting 12 h prior tothe tissue expression analysis. This stage is a critical period for cuticle development, as the locusts have just completed molting, and yolk formation has not yet commenced [47]. The experimental findings revealed the predominant expression of FoxO in the epidermal tissues, with relatively high expression levels observed in the ovaries (Figure 2A). As a downstream target gene of cellular nutrients, growth factors, and insulin signaling pathways (IIS), FoxO plays a regulatory role in physiological processes such as growth, development, and reproduction, including insect molting and metamorphosis. In Bombyx mori, the transcriptional levels of FOXO increase during the ecdysone hormone 20E-induced molting and pupation processes, highlighting its crucial involvement [48]. FOXO silencing in Helicoverpa armigera results in failed molting and the inhibition of the 20E signal gene expression, further confirming its necessity during insect molting and metamorphosis [49]. Therefore, we hypothesize that the primary function of FoxO in newly molted locusts lies in epidermal development rather than in ovarian development. However, the specific mechanism requires further investigation. Notably, the key genes associated with the Hippo pathway exhibited significantly higher expression levels, specifically within the female locust ovaries, compared to other tissues such as the brain, integument, and midgut (Figure 2B). Based on these findings, we predict that both FoxO and the Hippo signaling pathway play crucial roles in insect reproduction.
FoxO exerts an impact on reproduction in various insects [2,50,51]. In the mosquitos Culex pipiens and Aedes aegypt, FoxO knockdown represses Vg expression, leading to reduced reproductive rates [52,53]. The depletion of FoxO also suppresses Vg expression and diminishes ovarian development in the soybean pod borer Maruca vitrata [54]. Collectively, these studies support our observation that FoxO knockdown in vitellogenic female locusts significantly reduces Vg expression while impeding oocyte maturation and arresting ovarian growth (Figure 4). Following interference with the FoxO expression, the depletion of FoxO leads to a significant reduction in adipocyte polyploidy, accompanied by decreased Vg expression and impaired oocyte maturation, resulting in hindered ovarian growth in locusts [16]. Wu et al. provide evidence that FoxO is a crucial player in JH-dependent polyploidization, vitellogenesis, and egg development, which extends the view of JH action in insect cell polyploidization and vitellogenesis; however, the regulatory role of FoxO in insect vitellogenesis is not well defined.
Notch plays a crucial role in insect oogenesis [29,55,56]. The loss of function of Notch arrests the development of stalk and polar cells [57]. In L. migratoria, Notch-depleted adult females had blocked oocyte maturation and arrested ovarian growth [10]. This is consistent with our findings. In our study, we demonstrated that the dsFoxO treatment resulted in significantly decreased Notch expression levels (Figure 3B), accompanied by reduced Vg transcripts (Figure 4A), arrested oocyte maturation, and blocked ovarian growth (Figure 4B). Additionally, the Hippo pathway plays a crucial role in regulating the Notch receptor levels in follicle cells [29,30,58]. In D. melanogaster, the control of the mitosis–endocycle switch in follicular cells has been associated with the Notch pathway, as Notch signaling is attenuated in Hippo mutants [30,59]. In Drosophila imaginal discs, the Hippo pathway regulates membrane receptor trafficking, including the Notch receptor [60]. Our study demonstrated that the dsFoxO treatment resulted in significantly decreased levels of the key genes of Hippo (Figure 3B), accompanied by reduced Notch transcripts (Figure 3B) and suppressed reproduction (Figure 4). In the previous section, we demonstrated that the Hippo signaling pathway is one of the targets of FoxO in L. migratoria and that it promotes Notch signaling in the regulation of cell differentiation and proliferation, and oocyte polarity. Although we could not exclude the involvement of other potential signaling molecules, the findings in the present study, together with our previous analysis, suggest that FoxO regulates locust reproduction through Hippo–Notch.
Egg production is one of the most energy-demanding events in the adult lives of female insects. In addition to Vg, large amounts of carbohydrates and lipids are required to meet the energy demands of oocyte growth [61]. The insulin signaling pathway is involved in the regulation of the circulating sugar levels; thus, FoxO plays an important role in the regulation of sugar levels as a downstream target gene of the insulin signaling pathway [62]. It is obvious that the female reproductive processes require considerable amounts of energy-rich substrates and FoxO-mediated energy mobilization may be involved in the regulation of egg production [63,64]. In insects, trehalose accumulation primarily arises from glycogen breakdown metabolism [65,66,67]. The change pattern of the trehalose content is opposite to that of the glycogen content, which aligns with the experimental results obtained in this study. Our experiment revealed a significant decrease in the trehalose content (Figure 5B) after FoxO RNAi, while the glycogen content increased significantly (Figure 5A). Decreasing glycogen storage leads to a metabolic shift, resulting in increased internal trehalose [68]. Considering the dynamic fluctuations in the total sugar and glycogen contents, a reciprocal conversion between trehalose and glycogen may occur. Trehalose homeostasis regulates vitellogenesis and oocyte development in female insects. In L. migratoria and P. americanahe, trehalose involvement in Vg synthesis in the fat body and Vg uptake by the developing oocytes have been confirmed [69,70]. In our experiments, FoxO interference severely reduced the trehalose content, thus greatly reducing the synthesis and uptake of Vg in locusts disrupted by FoxO. This also demonstrates that FoxO-mediated energy mobilization is involved in the regulation of egg production.
5. Conclusions
Our study provides evidence that FoxO promotes fat body vitellogenesis in locusts through the Hippo signaling pathway–Notch. FoxO silencing results in decreased female locust reproduction. In addition, FoxO-mediated energy mobilization is involved in the regulation of egg production. These findings expand our understanding of the physiological functions of FoxO in insects and emphasize its significance in locust reproduction. Overall, these results highlight the potential of targeting FoxO as a novel molecular approach for controlling L. migratoria.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/insects15110891/s1, Supplementary File S1: The cDNA sequence and primers for FoxO clones and sequencing results; Figure S1: Heatmap depicting the increases in mRNA expression [46]; Figure S2: Interaction between FOXO protein and key proteins in Hippo pathway in Drosophila melanogaster; Table S1: Interactions between FoxO and Hpo, Sd, and Yki in Drosophila melanogaster.
Author Contributions
Conceptualization, J.X. and Z.Y.; methodology, J.X., Z.Y. and H.Z.; software, J.X. and N.C.; validation, J.X., H.Z., Z.Y. and X.W.; formal analysis, H.Z., T.M., J.X. and N.C.; investigation, B.T. and T.M.; data curation, B.T. and T.M.; writing—original draft preparation, J.X. and Z.Y.; writing—review and editing, J.X.; visualization, S.W. and G.C.; supervision, S.W.; project administration, S.W.; funding acquisition, S.W. and G.C. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China [Grant Nos. 30970473 and 31270459] and the Project of Zhejiang Qian-Jiang Talents Program [2010R10093].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sun X., Liu B.Q., Chen Z.B., Li C.Q., Li X.Y., Hong J.S., Luan J.B. Vitellogenin facilitates associations between the whitefly and a bacteriocyte symbiont. mBio. 2023;14:e02990-22. doi: 10.1128/mbio.02990-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy S., Saha T.T., Zou Z., Raikhel A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018;63:489–511. doi: 10.1146/annurev-ento-020117-043258. [DOI] [PubMed] [Google Scholar]
- 3.Mao Q., Wu W., Huang L., Yi G., Jia D., Chen Q., Chen H., Wei T. Insect bacterial symbiont-mediated vitellogenin uptake into oocytes to support egg development. mBio. 2020;11:e01142-20. doi: 10.1128/mBio.01142-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu S., Liu F., Zeng H., Li N., Ren C., Su Y., Zhou S., Wang G., Palli S.R., Wang J., et al. Insulin/IGF signaling and TORC1 promote vitellogenesis via inducing juvenile hormone biosynthesis in the American cockroach. Development. 2020;147:dev188805. doi: 10.1242/dev.188805. [DOI] [PubMed] [Google Scholar]
- 5.Jing Y.P., Wen X., Li L., Zhang S., Zhang C., Zhou S. The vitellogenin receptor functionality of the migratory locust depends on its phosphorylation by juvenile hormone. Proc. Natl. Acad. Sci. USA. 2021;118:e2106908118. doi: 10.1073/pnas.2106908118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth T.F., Porter K.R. Yolk protein uptake in the oocyte of the mosquito Aedes aegypti. J. Cell Biol. 1964;20:313–332. doi: 10.1083/jcb.20.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sappington T.W., Raikhel A.S. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol. 1998;28:277–300. doi: 10.1016/S0965-1748(97)00110-0. [DOI] [PubMed] [Google Scholar]
- 8.Ruohola H., Bremer K.A., Baker D., Swedlow J.R., Jan L.Y., Jan Y.N. Role of neurogenic genes in establishment of follicle cell fate and oocyte polarity during oogenesis in Drosophila. Cell. 1991;66:433–449. doi: 10.1016/0092-8674(81)90008-8. [DOI] [PubMed] [Google Scholar]
- 9.López-Schier H., St Johnston, D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001;15:1393–1405. doi: 10.1101/gad.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J., Li W., Zhao H., Zhou S. Clustered miR-2, miR-13a, miR-13b and miR-71 coordinately target Notch gene to regulate oogenesis of the migratory locust Locusta migratoria. Insect Biochem. Mol. Biol. 2019;106:39–46. doi: 10.1016/j.ibmb.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Hansen I.A., Attardo G.M., Rodriguez S.D., Drake L.L. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front. Physiol. 2014;5:103. doi: 10.3389/fphys.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S., Dong H.H. FoxO integration of insulin signaling with glucose and lipid metabolism. J. Endocrinol. 2017;233:R67–R79. doi: 10.1530/JOE-17-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu N., Wei S.F., Xu H.J. Transcriptome analysis of the regulatory mechanism of FoxO on wing dimorphism in the Brown Planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) Insects. 2021;12:413. doi: 10.3390/insects12050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greer E.L., Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Tang N., Hadden T.J., Rishi A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z., He Q., Zeng B., Zhou H., Zhou S. Juvenile hormone acts through FoxO to promote Cdc2 and Orc5 transcription for polyploidy-dependent vitellogenesis. Development. 2020;147:dev188813. doi: 10.1242/dev.188813. [DOI] [PubMed] [Google Scholar]
- 17.Huangfu N., Zhu X., Wang L., Zhang K., Li D., Chen L., Gao X., Niu L., Gao M., Ji J., et al. Insulin Receptor Substrate-1 (IRS1) Regulates oogenesis and vitellogenesis in Propylea japonica by mediating the FOXO transcription factor expression, independent of JH and 20E signaling pathways. J. Agric. Food Chem. 2023;71:300–310. doi: 10.1021/acs.jafc.2c07433. [DOI] [PubMed] [Google Scholar]
- 18.Süren-Castillo S., Abrisqueta M., Maestro J.L. FoxO inhibits juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 2012;42:491–498. doi: 10.1016/j.ibmb.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Xu H.J., Zhang C.X. Insulin receptors and wing dimorphism in rice planthoppers. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017;372:20150489. doi: 10.1098/rstb.2015.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domínguez C.V., Pagone V., Maestro J.L. Regulation of insulin-like peptide expression in adult Blattella germanica females. Insect Biochem. Mol. Biol. 2022;141:103706. doi: 10.1016/j.ibmb.2021.103706. [DOI] [PubMed] [Google Scholar]
- 21.Abrisqueta M., Süren-Castillo S., Maestro J.L. Insulin receptor-mediated nutritional signalling regulates juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem. Mol. Biol. 2014;49:14–23. doi: 10.1016/j.ibmb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Parthasarathy R., Palli S.R. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2011;41:294–305. doi: 10.1016/j.ibmb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma S., Meng Z., Chen R., Guan K.L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019;88:577–604. doi: 10.1146/annurev-biochem-013118-111829. [DOI] [PubMed] [Google Scholar]
- 24.Sayedyahossein S., Thines L., Sacks D.B. Ca2+ signaling and the Hippo pathway: Intersections in cellular regulation. Cell Signal. 2023;110:110846. doi: 10.1016/j.cellsig.2023.110846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kango-Singh M., Nolo R., Tao C., Verstreken P., Hiesinger P.R., Bellen H.J., Halder G. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129:5719–5730. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- 26.Udan R.S., Kango-Singh M., Nolo R., Tao C., Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 27.Harvey K.F., Pfleger C.M., Hariharan I.K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/S0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 28.Huang J., Wu S., Barrera J., Matthews K., Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Irles P., Piulachs M.D. Unlike in Drosophila meroistic ovaries, hippo represses notch in Blattella germanica panoistic ovaries, triggering the mitosis-endocycle switch in the follicular cells. PLoS ONE. 2014;9:e113850. doi: 10.1371/journal.pone.0113850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J., Poulton J., Huang Y.C., Deng W.M. The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS ONE. 2008;3:e1761. doi: 10.1371/journal.pone.0001761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meignin C., Alvarez-Garcia I., Davis I., Palacios I.M. The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr. Biol. 2007;17:1871–1878. doi: 10.1016/j.cub.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elshaer N., Piulachs M.D. Crosstalk of EGFR signalling with Notch and Hippo pathways to regulate cell specification, migration and proliferation in cockroach panoistic ovaries. Biol. Cell. 2015;107:273–285. doi: 10.1111/boc.201500003. [DOI] [PubMed] [Google Scholar]
- 33.Chapuis M.P., Lecoq M., Michalakis Y., Loiseau A., Sword G.A., Piry S., Estoup A. Do outbreaks affect genetic population structure? A worldwide survey in Locusta migratoria, a pest plagued by microsatellite null alleles. Mol. Ecol. 2008;17:3640–3653. doi: 10.1111/j.1365-294X.2008.03869.x. [DOI] [PubMed] [Google Scholar]
- 34.Sangbaramou R., Camara I., Huang X.Z., Shen J., Tan S.Q., Shi W.P. Behavioral thermoregulation in Locusta migratoria manilensis (Orthoptera: Acrididae) in response to the entomopathogenic fungus, Beauveria bassiana. PLoS ONE. 2018;13:e0206816. doi: 10.1371/journal.pone.0206816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Fang X., Yang P., Jiang X., Jiang F., Zhao D., Li B., Cui F., Wei J., Ma C., et al. The locust genome provides insight into swarm formation and long-distance flight. Nat. Commun. 2014;5:2957. doi: 10.1038/ncomms3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Tenlen J.R. Microinjection of dsRNA in Tardigrades. Cold Spring Harb. Protoc. 2018;11:prot102368. doi: 10.1101/pdb.prot102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlsson P., Mahlapuu M. Forkhead transcription factors: Key players in development and metabolism. Dev. Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- 39.Barthel A., Schmoll D., Unterman T.G. FoxO proteins in insulin action and metabolism. Trends Endocrinol. Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Clark K.L., Halay E.D., Lai E., Burley S.K. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 41.Santos B.F., Grenho I., Martel P.J., Ferreira B.I., Link W. FOXO family isoforms. Cell Death Dis. 2023;14:702. doi: 10.1038/s41419-023-06177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehtinen M.K., Yuan Z., Boag P.R., Yang Y., Villén J., Becker E.B., DiBacco S., de la Iglesia N., Gygi S., Blackwell T.K., et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 43.Mao B., Gao Y., Bai Y., Yuan Z. Hippo signaling in stress response and homeostasis maintenance. Acta Biochim. Biophys. Sin. 2015;47:2–9. doi: 10.1093/abbs/gmu109. [DOI] [PubMed] [Google Scholar]
- 44.Kudryashova T.V., Dabral S., Nayakanti S., Ray A., Goncharov D.A., Avolio T., Shen Y., Rode A., Pena A., Jiang L., et al. Noncanonical HIPPO/MST Signaling via BUB3 and FOXO drives pulmonary vascular cell growth and Survival. Circ. Res. 2022;130:760–778. doi: 10.1161/CIRCRESAHA.121.319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birnbaum A., Wu X., Tatar M., Liu N., Bai H. Age-dependent changes in transcription factor FOXO targeting in female Drosophila. Front. Genet. 2019;10:312. doi: 10.3389/fgene.2019.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding K., Barretto E.C., Johnston M., Lee B., Gallo M., Grewal S.S. Transcriptome analysis of FOXO-dependent hypoxia gene expression identifies Hipk as a regulator of low oxygen tolerance in Drosophila. G3. 2022;12:jkac263. doi: 10.1093/g3journal/jkac263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersen S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010;40:166–178. doi: 10.1016/j.ibmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Hossain M.S., Liu Y., Zhou S., Li K., Tian L., Li S. 20-Hydroxyecdysone-induced transcriptional activity of FoxO upregulates brummer and acid lipase-1 and promotes lipolysis in Bombyx fat body. Insect Biochem. Mol. Biol. 2013;43:829–838. doi: 10.1016/j.ibmb.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Cai M.J., Zhao W.L., Jing Y.P., Song Q., Zhang X.Q., Wang J.X., Zhao X.F. 20-Hydroxyecdysone activates Forkhead box O to promote proteolysis during Helicoverpa armigera molting. Development. 2016;143:1005–1015. doi: 10.1242/dev.128694. [DOI] [PubMed] [Google Scholar]
- 50.Koyama T., Mendes C.C., Mirth C.K. Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front. Physiol. 2013;4:263. doi: 10.3389/fphys.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santos C.G., Humann F.C., Hartfelder K. Juvenile hormone signaling in insect oogenesis. Curr. Opin. Insect Sci. 2019;31:43–48. doi: 10.1016/j.cois.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Sim C., Denlinger D.L. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. USA. 2008;105:6777–6781. doi: 10.1073/pnas.0802067105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansen I.A., Sieglaff D.H., Munro J.B., Shiao S.H., Cruz J., Lee I.W., Heraty J.M., Raikhel A.S. Forkhead transcription factors regulate mosquito reproduction. Insect Biochem. Mol. Biol. 2007;37:985–997. doi: 10.1016/j.ibmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al Baki M.A., Lee D.W., Jung J.K., Kim Y. Insulin signaling mediates previtellogenic development and enhances juvenile hormone-mediated vitellogenesis in a lepidopteran insect, Maruca vitrata. BMC Dev. Biol. 2019;19:14. doi: 10.1186/s12861-019-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Assa-Kunik E., Torres I.L., Schejter E.D., Johnston D.S., Shilo B.Z. Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development. 2007;134:1161–1169. doi: 10.1242/dev.02800. [DOI] [PubMed] [Google Scholar]
- 56.Volkova E.I., Dorogova N.V., Andreyenkov O.V., Tikhomirov S.A., Demakov S.A. New Mutations in the 5′ Region of the Notch Gene Affect Drosophila melanogaster Oogenesis. J. Dev. Biol. 2022;10:32. doi: 10.3390/jdb10030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen H.J., Wang C.M., Wang T.W., Liaw G.J., Hsu T.H., Lin T.H., Yu J.Y. The Hippo pathway controls polar cell fate through Notch signaling during Drosophila oogenesis. Dev. Biol. 2011;357:370–379. doi: 10.1016/j.ydbio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Polesello C., Tapon N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 2007;17:1864–1870. doi: 10.1016/j.cub.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 59.Deng W.M., Althauser C., Ruohola-Baker H. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development. 2001;128:4737–4746. doi: 10.1242/dev.128.23.4737. [DOI] [PubMed] [Google Scholar]
- 60.Maitra S., Kulikauskas R.M., Gavilan H., Fehon R.G. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr. Biol. 2006;16:702–709. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- 61.Ziegler R., Ibrahim M.M. Formation of lipid reserves in fatbody and eggs of the yellow fever mosquito, Aedes aegypti. J. Insect Physiol. 2001;47:623–627. doi: 10.1016/S0022-1910(00)00158-X. [DOI] [PubMed] [Google Scholar]
- 62.Broughton S., Alic N., Slack C., Bass T., Ikeya T., Vinti G., Tommasi A.M., Driege Y., Hafen E., Partridge L. Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE. 2008;3:e3721. doi: 10.1371/journal.pone.0003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lenaerts C., Monjon E., Van Lommel J., Verbakel L., Vanden Broeck J. Peptides in insect oogenesis. Curr. Opin. Insect Sci. 2019;31:58–64. doi: 10.1016/j.cois.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Leyria J., Orchard I., Lange A.B. The involvement of insulin/ToR signaling pathway in reproductive performance of Rhodnius prolixus. Insect Biochem. Mol. Biol. 2021;130:103526. doi: 10.1016/j.ibmb.2021.103526. [DOI] [PubMed] [Google Scholar]
- 65.Mariano A.C., Santos R., Gonzalez M.S., Feder D., Machado E.A., Pascarelli B., Gondim K.C., Meyer-Fernandes J.R. Synthesis and mobilization of glycogen and trehalose in adult male Rhodnius prolixus. Arch. Insect Biochem. Physiol. 2009;72:1–15. doi: 10.1002/arch.20319. [DOI] [PubMed] [Google Scholar]
- 66.Matsuda H., Yamada T., Yoshida M., Nishimura T. Flies without trehalose. J. Biol. Chem. 2015;290:1244–1255. doi: 10.1074/jbc.M114.619411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L., Wang H., Chen J., Shen Q., Wang S., Xu H., Tang B. Glycogen phosphorylase and glycogen synthase: Gene cloning and expression analysis reveal their role in trehalose metabolism in the brown planthopper, Nilaparvata lugens Stål (Hemiptera: Delphacidae) J. Insect Sci. 2017;17:42. doi: 10.1093/jisesa/iex015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seo Y., Kingsley S., Walker G., Mondoux M.A., Tissenbaum H.A. Metabolic shift from glycogen to trehalose promotes lifespan and healthspan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2018;115:E2791–E2800. doi: 10.1073/pnas.1714178115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lum P.Y., Chino H. Trehalose, the insect blood sugar, inhibits loading of diacylglycerol by lipophorin from the fat body in locusts. Biochem. Biophys. Res. Commun. 1990;172:588–594. doi: 10.1016/0006-291X(90)90714-X. [DOI] [PubMed] [Google Scholar]
- 70.Kono Y., Takahashi M., Mihara M., Matsushita K., Kameda Y. Effect of a trehalase inhibitor, validoxylamine a, on oocyte development and ootheca formation in Periplaneta americana (blattodea, blattidae) Appl. Entomol. Zool. 1997;32:293–301. doi: 10.1303/aez.32.293. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be made available upon request.