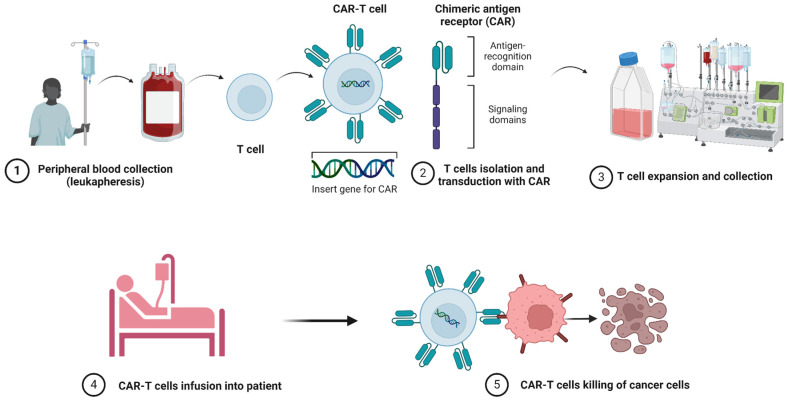
Figure 1.
Schematic representation of the CAR-T cell production process. Autologous chimeric antigen receptor (CAR) T cell therapy, which is currently approved, works by modifying a patient’s own T cells ex vivo and then administering them back to the same person. The production process is carried out in accordance with Good Manufacturing Practice (GMP) guidelines and is overseen by multiple quality assessments. The first step in autologous CAR-T cell production is the isolation of T cells from the patient’s peripheral blood. Isolated T cells are then activated and genetically engineered via viral transduction to express the CAR molecule. CAR-T cells are subsequently expanded in culture to up to billions of cells. The modified CAR-T cells are typically cryopreserved and later re-infused into the patient to specifically target and eliminate cancer cells. Created in BioRender. Gaimari, A. (2022).