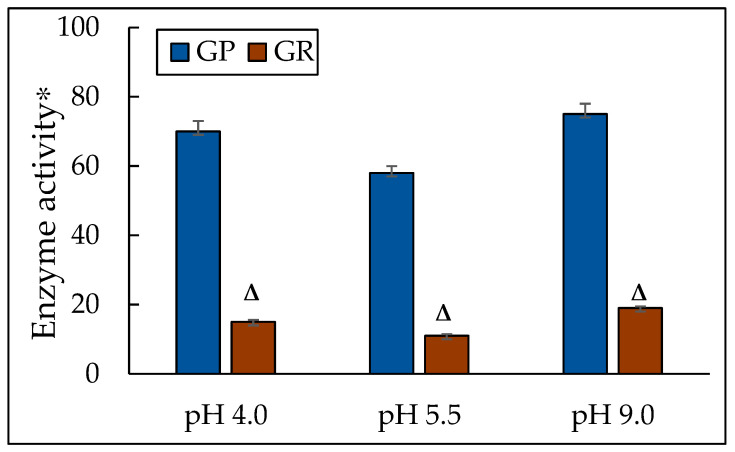
Figure 6.
The activity of glutathione peroxidase and glutathione reductase in the Y. lipolytica culture grown at different pHs. * Unit of enzymatic activity per 1 mg of protein. Cellular GPxs were measured at 340 nm in 0.05 mM K-Pi-buffer, pH 7.4; containing 1 mM EDTA, 0.12 mM NADP+, 0.85 mM GSH, 0.37 mM H2O2, and 1 unit of GR per mL. The same medium without glutathione served as the control. The reaction was initiated by the enzyme application. The rate of NADP+ reduction due to enzyme reactions such as oxidized glutathione formation by GPxs action followed by its reduction resulting from NADP+ oxidation by GR action was monitored by a decrease in A. One unit of enzyme (EU) activity was defined as the enzyme amount catalyzing 1 µmol final reaction product at +25 °C per min. The calculation is the same as that for IDH. Δ—statistically significant difference compared to the corresponding control, p < 0.005. Values are mean ± S.E.M. from 5–6 independent experiments.