FIG. 1.
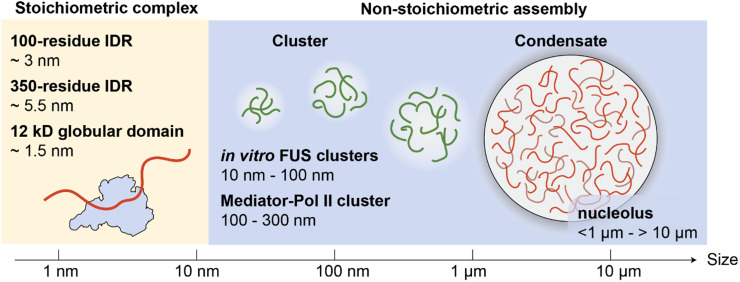
Assemblies of intrinsically disordered regions (IDRs) at various scales. IDRs form stoichiometric complexes with defined molar ratios. For example, an IDR with ∼100 residues has a radius of gyration (Rg) of approximately 3 nm,29 an IDR with ∼350 residues has an Rg of approximately 5.5 nm,30 and a 12 kD globular protein domain has an Rg of approximately 1.5 nm.31 IDRs also form non-stoichiometric assemblies, where the protein molar ratio is not fixed. For example, below the saturation concentration for liquid-liquid phase separation (LLPS), FUS proteins form clusters ranging from 10 to 100 nm in size,32 and Mediator-RNA clusters in cells range from 100 to 300 nm.33 IDR condensates formed through LLPS are larger, with membraneless organelles such as the nucleoli ranging in size from less than 1 μm to over 10 μm.34