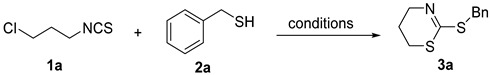
Table 1.
Screening of the thiol-involved cascade reaction a.
| |||||
|---|---|---|---|---|---|
| Entry | Base (eq.) | Solvent | Temp. (°C) | Time (min) | 3a (%) b |
| 1 | K2CO3 (0.6) | neat | 25 | 60 | 30 |
| 2 | K2CO3 (0.6) | H2O | 25 | 60 | 49 |
| 3 | K2CO3 (0.6) | EtOH | 25 | 60 | 31 |
| 4 | K2CO3 (0.6) | EtOH/H2O | 25 | 60 | 78 |
| 5 | K2CO3 (0.6) | EtOH/H2O | 50 | 60 | 96 |
| 6 | K2CO3 (0.6) | EtOH/H2O | 50 | 20 | 95 |
| 7 | NaOH (1.2) | EtOH/H2O | 50 | 20 | 94 |
| 8 | Na2CO3 (0.6) | EtOH/H2O | 50 | 20 | 94 |
| 9 | NaHCO3 (1.2) | EtOH/H2O | 50 | 20 | 92 |
| 10 | Et3N (1.2) | EtOH/H2O | 50 | 20 | 94 |
| 11 | DBU (1.2) | EtOH/H2O | 50 | 20 | 94 |
| 12 | K2CO3 (0.6) | Acetone | 50 | 20 | 91 |
| 13 | K2CO3 (0.5) | EtOH/H2O | 50 | 20 | 92 |
| 14 | K2CO3 (0.75) | EtOH/H2O | 50 | 20 | 95 |
| 15 c | K2CO3 (0.6) | EtOH/H2O | 50 | 5 | 99 |
| 16 c | K2CO3 (0.6) | EtOH/H2O | 80 | 5 | 95 |
| 17 d | K2CO3 (0.6) | EtOH/H2O | 50 | 5 | 98 |
a Reaction conditions: 1a (0.2 mmol), 2a (0.2 mmol), the loading of base based on 1a. EtOH (1 mL), H2O (1 mL); b isolated yields; c MW, 20 W; d using 3-iodopropyl isothiocyanate, MW, 20 W.
