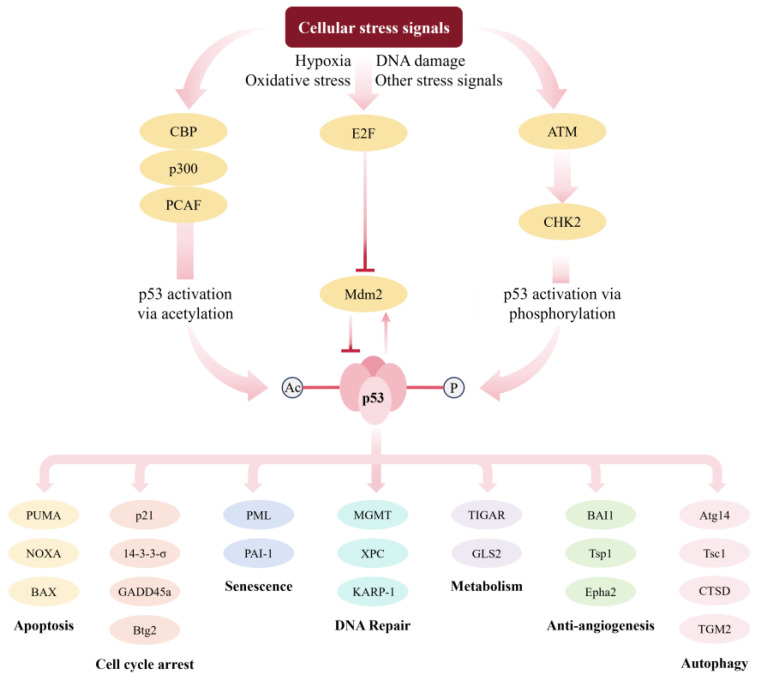
Figure 1.
The activation and regulation of p53. Mdm2 can directly bind to p53, inhibiting its transcriptional activity. This interaction leads to the ubiquitination and proteasomal degradation of p53 as well as its export from the nucleus. The overactivity of Mdm2 may reduce the efficiency of p53 translated from a transfected vector. When cells are exposed to various stress signals, such as hypoxia, oxidative stress, or DNA damage, the pathways for p53 acetylation or phosphorylation are activated. This inhibits Mdm2-mediated ubiquitination and degradation of p53, promoting its stability and accumulation. Under pressure signals, E2F1 inhibits the expression of Mdm2, a ubiquitin ligase that promotes the degradation of p53. p300/CBP and related protein PCAF can bind and acetylate p53. The ATM activates its kinase activity, followed by phosphorylation and activation of CHEK2, which further phosphorylates the p53 protein. The phosphorylation and acetylation of p53 can inhibit its ubiquitin degradation and promote the stability and accumulation of p53. Then, p53 triggers cell apoptosis, cell cycle arrest, cell senescence, DNA repair, metabolic regulation, anti-tumor angiogenesis, and autophagy by regulating various downstream target genes or interactions with other proteins.