Abstract
The global spread of antimicrobial resistance genes (ARGs) poses a significant threat to public health. While antibiotics effectively treat bacterial infections, they can also induce gut dysbiosis, the severity of which varies depending on the specific antibiotic treatment used. However, it remains unclear how gut dysbiosis affects the mobility and dynamics of ARGs. To address this, mice were pre-treated with streptomycin, ampicillin, or sulfamethazine, and then orally inoculated with Salmonella enterica serovar Typhimurium and S. Heidelberg carrying a multi-drug resistance IncA/C plasmid. The streptomycin pre-treatment caused severe microbiome perturbation, promoting the high-density colonization of S. Heidelberg and S. Typhimurium, and enabling an IncA/C transfer from S. Heidelberg to S. Typhimurium and a commensal Escherichia coli. The ampicillin pre-treatment induced moderate microbiome perturbation, supporting only S. Heidelberg colonization and the IncA/C transfer to commensal E. coli. The sulfamethazine pre-treatment led to mild microbiome perturbation, favoring neither Salmonella spp. colonization nor a conjugative plasmid transfer. The degree of gut dysbiosis also influenced the enrichment or depletion of the ARGs associated with mobile plasmids or core commensal bacteria, respectively. These findings underscore the significance of pre-existing gut dysbiosis induced by various antibiotic treatments on ARG dissemination and may inform prudent antibiotic use practices.
Keywords: antimicrobial resistance, plasmid transfer, gut microbiome, antibiotic treatments, Salmonella
1. Introduction
The global spread of antimicrobial resistance (AMR) genes (ARGs) among pathogenic bacteria constitutes a severe public health concern, posing a significant threat to the effective treatment of an expanding array of bacterial infections [1]. The excessive and inappropriate use of antimicrobials in animal production has led to the widespread prevalence of antimicrobial-resistant bacteria (ARB) in agricultural systems and the environment [2,3,4]. Numerous governments and organizations have adopted a One Health approach, striving to reduce the risk of AMR transmission to humans through food consumption and environmental exposure [5,6,7]. To realize this objective, it is crucial to understand the dynamics of food- or water-borne ARB and the factors that might influence the potential dissemination of their ARGs to the pathogenic and commensal bacteria within a host’s gut microbiota.
Gut microbiota are the community of microorganisms that coexist symbiotically within a host’s digestive tract, playing a vital role in maintaining the host’s metabolic homeostasis, regulating their immune system, and influencing their susceptibility to pathogens [8]. Additionally, they serve as a reservoir for diverse antimicrobial resistance genes and determinants, collectively referred as the resistome [9]. Various factors, such as genetic background, diet, lifestyle, and antibiotic treatment, can impact the composition of a gut microbiome and its resistome [9,10]. Specifically, antibiotic treatment has the potential to disrupt the balance of a gut microbiome, reduce microbial diversity, foster the overgrowth of opportunistic pathogens, modify metabolic functions, and weaken the immune response [11].
Of greater concern is the fact that the use of antibiotics may facilitate the emergence of antibiotic-resistant bacteria in the gut. Predictions based on three-dimensional protein structures indicated that gut bacteria might harbor over 6000 uncharacterized AMR determinants, with the majority intrinsic to dominant commensals and seldom shared with bacterial pathogens [12]. However, a recent metagenomics analysis demonstrated that mobile genetic elements played a crucial role in mediating ARG acquisition in gut commensals following a broad-spectrum antibiotic treatment in a murine model [13]. Moreover, a systematic study evaluating the impact of 144 different antibiotics on gut bacteria revealed that β-lactam resistance among gut commensals was strain-specific and likely associated with horizontal gene transfer [14]. Given the variability in antibiotic treatments, encompassing the specific antibiotics used, dosage, and duration of the treatment, a more comprehensive understanding is essential to elucidate how antibiotics may influence the dissemination of ARGs within a gut microbiota.
Plasmid conjugation stands as a primary mechanism for horizontal gene transfer in bacteria, facilitating the exchange of genetic material through direct cell-to-cell contact between two bacterial cells. Conjugative plasmids play a significant role in the dissemination of ARGs in bacteria [15]. Through direct exposure, antibiotics may act as selective drivers of ARB, influencing the dynamics and efficiency of conjugation [16,17,18]. Moreover, through indirect pre-exposure, heavy antibiotic dosages (e.g., one oral dose of streptomycin at 1 g kg−1) can disrupt a gut microbiota, promoting the colonization and expansion of ARB and fostering conjugation [19,20]. Although the pre-exposure to therapeutic dosages of antibiotics enhances the colonization of bacterial pathogens [21], whether and how it facilitates a conjugative transfer of ARGs remains unclear. We hypothesized that the ARG dissemination would be positively associated with the levels of microbiome perturbation induced by the pre-exposure to antibiotics. Thus, we utilized a murine model, administering a heavy dose of streptomycin [19,20] as a positive control to induce severe gut dysbiosis, a clinical dosage of ampicillin, and a veterinary dosage of sulfamethazine to induce intermediate levels of gut dysbiosis, with no antibiotic as the negative control. Subsequently, we infected the mice with Salmonella enterica serotype Heidelberg (S. Heidelberg) as a donor of a multi-drug resistance IncA/C plasmid, and S. Typhimurium as a recipient. The present study involved a comparative analysis of the dynamics of the donor, recipient, transconjugant, and selected ARGs and an integrase gene (intI1), alongside an examination of the gut microbial composition. The ARGs chosen herein included a sulfonamide resistance gene (sul1), a streptomycin resistance gene (strA), a β-lactam resistance gene (cfxA), a macrolide, lincosamide, a streptogramin B resistance gene (ermF), and a tetracycline resistance gene (tetQ). The sul1 and strA genes, carried by the IncA/C plasmid [18], served as representatives of the introduced ARGs that were associated with mobile gene elements, whereas the cfxA, ermF, and tetQ genes were representatives of the ARGs highly prevalent in the gut commensal bacteria [22,23]. The intI1 gene, also carried by the IncA/C plasmid, was one of the key players mediating ARG dissemination [13].
2. Materials and Methods
2.1. Bacteria
Salmonella enterica serotype Heidelberg (SL-312) was isolated from a Canadian chicken farm. It contains a conjugative IncA/C plasmid, encoding the genes aph(3)-Ia, aph(3)-Ib or strA, aph(6)-Id or strB, blaTEM-1B, blaCMY-2, dfrA1, floR, sul1, sul2, tetA, and intI1. This S. Heidelberg isolate exhibits resistance to amoxicillin/clavulanic acid, ampicillin, cefazolin, cefoxitin, cefpodoxime, ceftiofur, cephamycin, chloramphenicol, streptomycin, sulfamethizole, tetracycline, and trimethoprim-sulphamethoxazole [18]. In the present study, S. Heidelberg served as the donor of the multi-drug resistance IncA/C plasmid. The recipient, Salmonella enterica serotype Typhimurium (SL1344), contains a mobile IncQ plasmid, encoding the strA, strB, and sul2 genes. To facilitate the recovery of S. Typhimurium, a spontaneous rifampicin-resistant mutant was generated. Briefly, S. Typhimurium was cultured overnight in Luria–Bertani (LB; Miller formulation, Difco, Fisher Scientific, Ottawa, ON, Canada) broth at 37 °C. A 1.0 mL aliquot of the overnight culture was pelleted, re-suspended in 100 µL LB broth, and spread on LB agar supplemented with 50 µg mL−1 rifampicin (LB-Rif). After 24 h of incubation, resistant colonies were selected and sub-cultured on LB-Rif agar to generate and maintained a S. Typhimurium rifampicin-resistant mutant culture.
2.2. In Vitro Conjugation
In vitro conjugation between S. Heidelberg (donor) and S. Typhimurium (recipient) was assessed following the method described by Laskey et al. [18]. Briefly, the donor and recipient strains were cultured separately in LB broth with shaking at 30 °C overnight. The cultures were pelleted and washed, and the cells were suspended to a final OD600 of 1.0 in 10−1 × LB broth. Donor and recipient cell suspensions were mixed in a 9:1 ratio and incubated statically overnight at 30 °C for conjugation. The enumeration of the donor, recipient, and transconjugant bacteria was performed using XLT4 agar (Difco, Fisher Scientific, Ottawa, ON, Canada) supplemented with 50 µg mL−1 ampicillin (XLT4-Amp); 50 µg mL−1 rifampicin (XLT4-Rif); and 50 µg mL−1 ampicillin and 50 µg mL−1 rifampicin (XLT4-Amp-Rif), respectively. The frequency of conjugation was quantified as the ratio of transconjugant to donor bacteria counted at the end of the mating incubation period.
2.3. In Vivo Conjugation
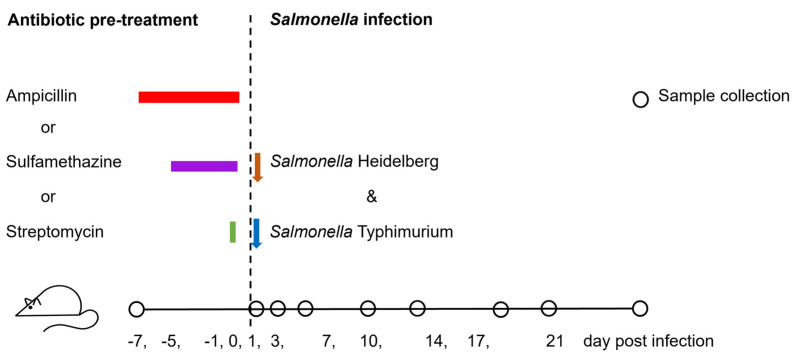
The mouse experiments and procedures, in compliance with the guidelines and ethical standards of the Canadian Council on Animal Care and the Animal Research: Reporting of In Vivo Experiments (ARRIVE), were approved by the Animal Care Committee at the Ottawa Laboratory (Fallowfield) of the Canadian Food Inspection Agency (Ottawa, ON, Canada). Female C57BL/6 mice, 28 days old, were acquired from Charles River Laboratories (Saint Constant, QC, Canada). The mice were mixed and acclimatized for two weeks before receiving antibiotic treatments. They were then housed two or three per cage (Optimice®, Animal Care Systems, Englewood, CO, USA) with water and feed provided ad libitum. A total of 21 mice were randomly divided into four groups for antibiotic pre-treatments: no antibiotic, ampicillin, streptomycin, and sulfamethazine (Table 1). Twenty-four hours after antibiotic withdrawal, the mice were first inoculated with the recipient bacteria and then with the donor bacteria one hour later. Bacterial inocula, consisting of 100 µL of a log-phase culture with approximately 3.0 × 108 colony forming units (CFUs) of either the recipient or donor bacteria in a phosphate buffered saline (PBS, pH 7.2), were administrated via oral gavage. The schedule of the experimental procedures is depicted in Figure 1. On designated sampling days, the mice were weighed and monitored for clinical symptoms like a ruffled coat, a hunched posture, and lethargy. Animals were euthanized upon the development of morbidity, defined as showing clinical symptoms and/or experiencing a weight loss exceeding 20% from a week before the bacterial inoculation. Fecal pellets were collected from all live mice on −7 (baseline)-, 0 (day of bacterial inoculation)-, 1-, 3-, 7-, 10-, 14-, 17-, and 21-days post-infection (DPI). These fecal pellets were processed as described by Laskey et al. [18] for DNA extraction and bacterial enumeration. Briefly, pellets were weighed and homogenized in 1.0 mL PBS. The homogenates were 10-fold serially diluted in PBS and suspensions were plated on three selective agars, XLT4 agar supplemented with 50 µg mL−1 ampicillin and 50 µg mL−1 tetracycline (XLT4-Amp-Tet); 50 µg mL−1 rifampicin and 50 µg mL−1 streptomycin (XLT4-Rif-Strep); and 50 µg mL−1 ampicillin, 50 µg mL−1 rifampicin, and 50 µg mL−1 streptomycin (XLT4-Amp-Rif-Strep), to enumerate the donor, recipient, and putative S. Typhimurium transconjugant, respectively. The limit for bacterial enumeration was 2.2 log10 CFU g−1 in feces. Additionally, Chromocult agar (EMD Millipore, Toronto, ON, Canada) supplemented with 50 µg mL−1 ampicillin (Chr-Amp) was used to isolate the potential commensal Enterobacteriaceae transconjugants.
Table 1.
Treatment groups in mouse experiments.
| Group | Antibiotic Pre-Treatment 1 | n |
|---|---|---|
| None | Control (no antibiotic) | 5 |
| Amp | Ampicillin, 40 mg kg−1 d−1 via drinking water for 7 days | 6 |
| Strep | Streptomycin, one dose of 20 mg per mouse via oral gavage | 5 |
| Sulf | Sulfamethazine, 225 mg kg−1 d−1 via drinking water for 5 days | 5 |
1Antibiotic pre-treatment was stopped 24 h before bacterial inoculation in all cases.
Figure 1.
Schedule of procedures for mice inoculated with Salmonella Typhimurium (recipient) and Salmonella Heidelberg (donor) following pre-treatment with either ampicillin, sulfamethazine, streptomycin, or no antibiotic. Sample collection was performed on various days post-infection (DPI) as indicated. Colored bars represent the duration of antibiotic pre-treatments, and arrows indicate the points of bacterial inoculation during the co-infection phase.
2.4. Whole Genome Sequencing
To identify putative transconjugant bacteria, the colonies displaying distinct morphologic characteristics on XLT4-Amp-Rif-Strep and Chr-Amp media (3 colonies each morphologic type) were isolated for each mouse. These isolates were sub-cultured on LB agar to generate single-isolated colonies. DNA was extracted from these isolates using the automated Qiagen EZ1 DNA tissue kit, according to the manufacturer’s instructions. The extracted DNA was subjected to polymerase chain reactions (PCRs) using primers for invA, blaCMY-2, and blaTEM-1 genes (Table S1). Isolates that tested negative for the invA gene but positive for both the blaCMY-2, and blaTEM-1 genes were selected for whole-genome sequencing (WGS). Isolates of the S. Heidelberg donor and S. Typhimurium recipient and some ampicillin-resistance bacteria were sequenced as well. The WGS was performed on the Illumina MiSeq system (Illumina Canada, Vancouver, BC, Canada). The library preparation was performed using the Illumina DNA Prep Kit (Illumina). This process yielded paired-end reads of 300 base pairs (bp). Genome assembly and analysis was performed using COWBAT v0.5.0.23 (https://github.com/OLC-Bioinformatics/COWBAT), a comprehensive tool integrating several steps such as quality control (QC) trimming with BBMap v38.96 (https://sourceforge.net/projects/bbmap/), assembly with SKESA v2.1 [24], quality assessment with QUAST v5.1.0 [25], and plasmid identification with MOB-suite v 3.0.3 [26].
2.5. 16S rRNA Gene Amplicon Sequencing
The DNA samples extracted from mouse fecal pellets using the NucleoSpin Soil DNA Extraction Kit (Macherey-Nagel, Dueren, Germany), underwent 16S rRNA gene amplicon sequencing. Briefly, the V3-V4 region of the 16S ribosomal RNA gene was amplified using PCR [27]. The sequencing libraries were prepared using the Nextera XT Index Kit v2 (Illumina), and then purified and normalized with the NGS Normalization 96-well kit (Norgen Biotek, Thorold, ON, Canada). Sequencing was performed on the Illumina MiSeq system using the MiSeq v3 kit with a 10% PhiX spike-in, targeting an output of 100,000 raw reads per sample. QC filtration of the raw reads was performed using Fastp v 0.23.2 [28], and primer sequences were removed using pTrimmer v 1.3.4 [29]. The processed reads were then classified with Emu v 3.4.5 with the pre-built Emu database for accurate microbial identification [30]. Data analysis and visualization were performed using the following R packages: vegan v 2.6-2, ggplot2 v 3.3.6, phyloseq v 1.38.0, and microbiomeMarker v 1.02.
2.6. Quantification of Antimicrobial Resistance and Integrase Genes
The abundance of strA, sul1, intI1, cfxA, emrF, tetQ, and the small subunit ribosomal RNA fragment 1 gene (rrnS1) was determined using a quantitative polymerase chain reaction (qPCR) with a QuantStudio 3 Real-Time PCR System (Thermo Fisher, Nepean, ON, Canada). The reaction mixture, with a total volume of 12.5 µL, contained 6.25 µL of Power SYBR Green PCR master mix (Thermo Fisher), 0.625 µL each of forward and reverse primers (primer sequences and final concentrations are detailed in Table S1), 2.5 µL of template DNA normalized to 0.4 ng µL−1, and 2.5 µL of nuclease free water. The PCR program included an initial incubation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s, with annealing and elongation set at specific temperatures and times (Table S1) [31,32,33,34,35,36,37,38,39,40]. A final melting curve analysis, ramping the temperature from the elongation setting to 95 °C, verified the specificity of the PCRs. Each DNA sample underwent triplicate PCR reactions. Standards for qPCR were prepared by synthesizing the sequences of all target amplicons and cloning them into a pUC-IDT plasmid (IDT, Coralville, IA, USA). The recombinant plasmid was transformed into an Escherichia coli DH-5α strain for amplification, and then extracted from the bacterial cells. The plasmid DNA was linearized, quantified, and used as standards for the construction of the qPCR standard curves. Detection limits for the various gene targets were 4.3 ± 0.6 log10 copies per gram of feces.
2.7. Statistical Analyses
Differences in conjugation frequency, mean abundance of each target bacterium, fold change of each targeted gene, and the relative abundance of each phylum, family, or genus in the 16S rRNA gene community profiles between the treatment and control groups on identical sampling days were analyzed with Welch’s ANOVA followed by Dunnett’s test, using GraphPad Prism 8.0 software (GraphPad Software, Boston, MA, USA). All correlations were tested using the Pearson correlation test. The treatment groups contained 5 or 6 mice (Table 1), and the mean value derived from the technical replicates of a fecal pellet from each mouse on every sampling day constituted one data point. A p-value < 0.05 was considered statistically significant. Linear discriminant analysis (LDA) effect size (LEfSe) [41] was used to identify the taxa that best discriminated one treatment group from the others on day 0 post-infection.
3. Results
3.1. Salmonella Colonization and Plasmid Conjugation
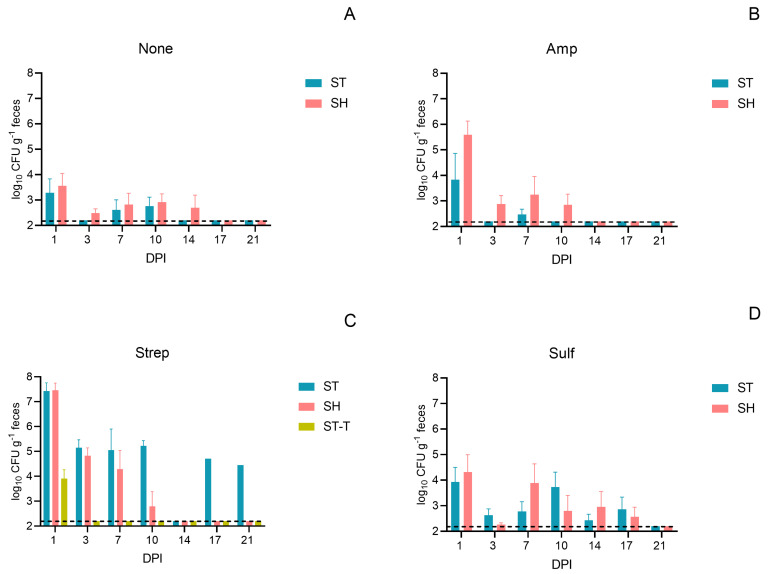
In the mice co-infected with S. Heidelberg (donor) and S. Typhimurium (recipient), fecal shedding of both bacterial strains showed similar patterns at most post-infection time points within each antibiotic pre-treatment group (Figure 2A–D). Both strains peaked at 1 DPI, and this gradually decreased to undetectable levels in most mice over the 21 days post-infection. Notably, mice pre-treated with streptomycin exhibited a significantly higher abundance of both Salmonella strains compared to the control that received no antibiotic pre-treatment (Figure 2A–D and Figure S1). On 1 DPI, both S. Heidelberg and S. Typhimurium reached a peak abundance of 7.4 log10 g−1, while the S. Typhimurium transconjugant reached 3.9 log10 g−1. The observed in vivo conjugation frequency, 6.5 (±3.3) ×10−4, was much higher than the in vitro conjugation frequency, 4.9 (±3.7) ×10−6 (p < 0.05). Additionally, Escherichia coli (E. coli) transconjugants were recovered from these mice (Table 2 and Table S2). In the mice pre-treated with ampicillin, S. Heidelberg showed a peak abundance at 5.6 log10 g−1, while S. Typhimurium did not exceed 3.8 log10 g−1. In this group, only E. coli transconjugants were detected, with no S. Typhimurium transconjugants recovered (Table 2 and Table S2). In contrast, the mice that received sulfamethazine or no antibiotic pre-treatment maintained the Salmonella strains at levels below 4.3 log10 g−1 throughout the 21-day post-infection period, with no transconjugants being recovered. Isolates of the donor, recipient, transconjugants, and some ampicillin-resistance bacteria underwent WGS. The sequencing data confirmed that the IncA/C plasmid was transferred from the S. Heidelberg donor to the S. Typhimurium recipient and the commensal E. coli. Additionally, the sequencing data confirmed the presence of the IncQ plasmid, encoding the strA, strB, and sul2 genes, in S. Typhimurium (SAMN40034283), and also showed intrinsic ARGs in some ampicillin-resistance commensal bacteria, such as the mdfA and blaACT genes in the chromosomes of E. coli (SAMN40034292) and Enterobacter xiangfangensis (SAMN40034293), respectively.
Figure 2.
Abundance (mean ± SE) of Salmonella Heidelberg (SH, donor), S. Typhimurium (ST, recipient), and S. Typhimurium transconjugants (ST-T) in fecal samples from mice that received both the recipient and donor inoculation following different pre-treatments. The pre-treatments include no antibiotic (None, A), ampicillin (Amp, B), streptomycin (Strep, C), and sulfamethazine (Sulf, D). The y-axis shows the logarithmic scale of the colony-forming units per gram of feces (log10 CFU g−1), and the x-axis denotes the days post-infection (DPI). The dash lines represent the detection limit of 2.2 log10 CFU g−1.
Table 2.
Transconjugants isolated from mice subjected to antibiotic pre-treatments followed by Salmonella infection.
| Pre-Treatment | Transconjugant Isolated (Day Post-Infection) 1 |
Accession Number 2 |
|---|---|---|
| None | Not detected | |
| Amp | Escherichia coli (1) | SAMN40034289-292 |
| Strep | Salmonella Typhimurium (1) | SAMN40034283-288 |
| Escherichia coli (1, 3) | SAMN40034294-297, SAMN40034299-303 | |
| Sulf | Not detected |
1 All transconjugants containing the IncA/C plasmid were confirmed via whole genome sequencing. 2 BioSample accession number of representative isolates.
3.2. Mouse Survival Post Salmonella Infection
All mice that received ampicillin or no antibiotic pre-treatment survived for a minimum of 21 days following co-infection with S. Typhimurium and S. Heidelberg (Figure S2, Table S3). In the group pre-treated with sulfamethazine, one out of five mice died on 1 DPI, while the remaining four mice survived the 21-day post-infection period. In contrast, the survival rates were significantly lower (p < 0.05) among the mice that received the streptomycin pre-treatment when compared to the other groups. Specifically, four out five mice with the streptomycin pre-treatment were euthanized due to more than 20% weight loss, two on 7 DPI, and two on 10 DPI. Only one mouse in the streptomycin pre-treatment group survived the 21-day post-infection period.
3.3. Dynamics of Antimicrobial Resistance and Integrase Genes
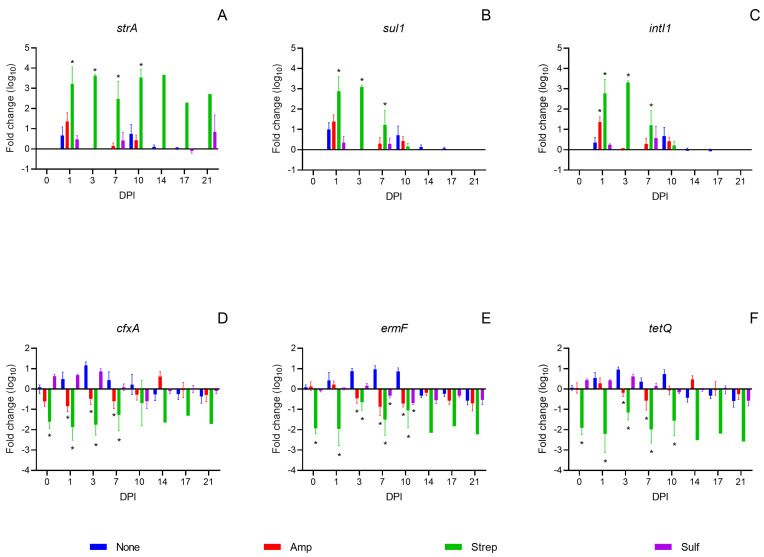
The antibiotic pre-treatments did not cause direct impacts on the dynamics of the strA, sul1, and intI1 genes, that were carried by the IncA/C plasmid. On 0 DPI, the abundance of these three genes remained unchanged across all mice, regardless of the pre-treatment (Figure 3A–C). However, following co-infection with the Salmonella donor and recipient, a noticeable increase in the abundance of all three genes was observed on 1 DPI in most mice. The mice that received a streptomycin pre-treatment showed a significant enrichment of these genes (p < 0.05) compared to those without an antibiotic pre-treatment from 1 to 7 DPI. Furthermore, the abundance of the sul1 and intI1 genes was positively correlated with that of S. Heidelberg (Pearson’s correlation coefficient rsul1 = 0.7229, rintI1 = 0.6580, Figure S3). The elevated levels of the sul1 and intI1 genes observed initially declined by 21 DPI across all the mice (Figure 3B,C). The abundance of the strA gene was positively correlated with that of Salmonella spp. (rstrA = 0.5816, Figure S3), as both the donor and recipient carry the strA gene. The strA abundance remained elevated in the streptomycin pre-treatment group due to the persistence of S. Typhimurium during the 21-day post-infection period (Figure 3A and Figure 2C).
Figure 3.
Fold change in the abundance of antimicrobial resistance genes strA, sul1, cfxA, tetQ, and ermF, and an integrase gene, intI1, (mean + SE) in fecal samples from mice following an inoculation with Salmonella Typhimurium and Salmonella Heidelberg. Mice were pre-treated with either no antibiotic (blue), ampicillin (red), streptomycin (green), or sulfamethazine (purple). Gene abundances were averaged from triplicate technical replicates and were normalized against that of the rrnS1 genes for each mouse. Fold changes from 0 to 21 days post-infection (DPI) were calculated relative to the baseline of −7 DPI. Asterisks (*) indicate a significant difference (p < 0.05) between an antibiotic pre-treatment group and the control group with no antibiotic pre-treatment within each DPI, as determined by Welch’s ANOVA followed by Dunnett’s test.
In comparison, the antibiotic pre-treatments caused direct impacts on the dynamics of the cfxA, ermF and tetQ genes, the representative ARGs carried by commensal bacteria. The abundance of these three genes significantly (p < 0.05) decreased on 0 DPI, and the decrease remained up to 10 DPI in the mice receiving the streptomycin pre-treatment (Figure 3D–F). In the mice pre-treated with ampicillin and sulfamethazine, the cfxA gene showed minor fluctuations, while the ermF and tetQ genes were relatively stable on 0 DPI. However, post Salmonella co-infection, the abundance of the cfxA, ermF and tetQ genes significantly decreased on various DPIs in the mice receiving the ampicillin pre-treatment. Whereas, in the sulfamethazine treatment group, the abundance of these genes fluctuated in a way similar to that of the mice receiving no antibiotic pre-treatment (Figure 3D–F).
3.4. Association Between a Gut Microbiome and the Dynamics of Target Genes
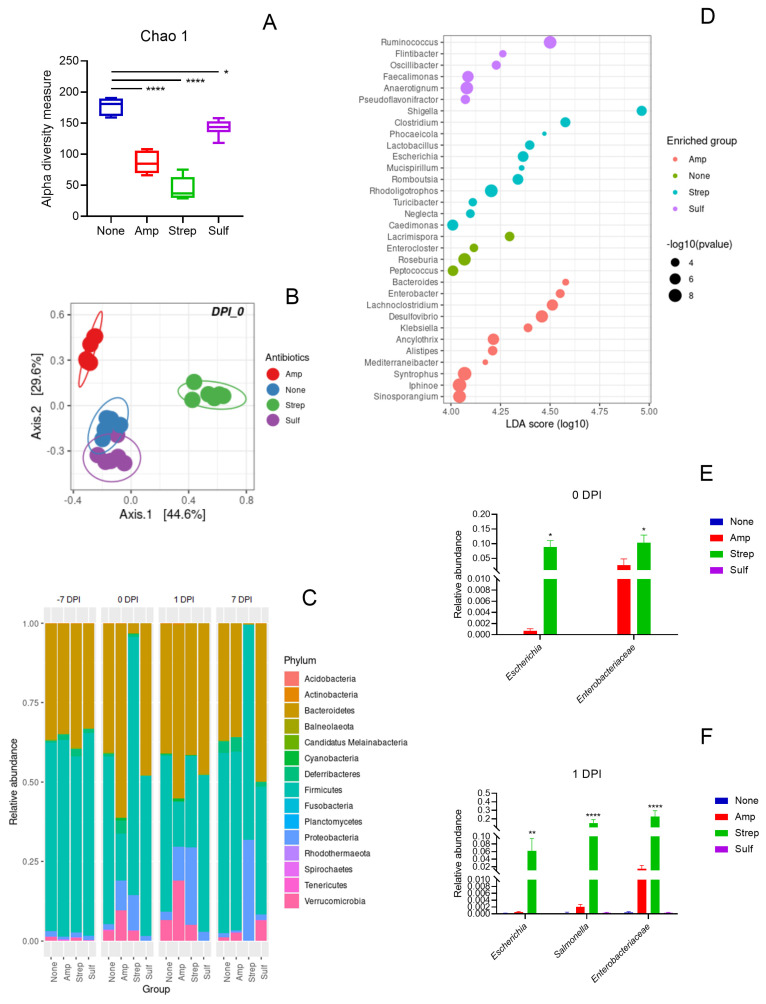
To investigate the association between a gut microbiome and the dynamics of target genes, the taxonomic composition of gut microbial communities was analyzed using 16S rRNA gene amplicon sequencing. All three antibiotic pre-treatments significantly reduced the species richness within each gut microbiome compared to the control, with the streptomycin pre-treatment causing the greatest reduction, followed by the ampicillin and the sulfamethazine pre-treatments (Figure 4A). The dissimilarity of the gut microbiomes between each treatment group and the control also reflected the different levels of perturbation induced by the antibiotic pre-treatments (Figure 4B). The gut microbiomes gradually recovered by 7 DPI in the mice receiving the ampicillin and the sulfamethazine pre-treatments, but not the streptomycin pre-treatment (Figures S4 and S5). The antibiotic pre-treatments caused differential impacts on the microbial compositions of the mice. The relative abundance of Proteobacteria increased on 0 DPI following the ampicillin and streptomycin pre-treatments compared to the no antibiotic control (Figure 4C). Post Salmonella infection, the relative abundance of Proteobacteria continued to rise with this increase persisting until at least 7 DPI in the mice that received the streptomycin pre-treatment. In comparison, the relative abundance of Proteobacteria in the mice that received the ampicillin pre-treatment returned to levels comparable to the no antibiotic control group on 7 DPI. The relative abundance of Proteobacteria in the mice pre-treated with sulfamethazine was similar to that of the no antibiotic control group from −7 to 7 DPI. Furthermore, the relative abundance of Escherichia and Enterobacteriaceae increased following the streptomycin and ampicillin pre-treatments on 0 and 1 DPI (Figure 4E,F).
Figure 4.
Analysis of the mouse gut microbiomes using 16S rRNA gene amplicon sequencing. (A) microbial species richness assessed by the Chao1 index on 0 days post-infection (DPI) following various antibiotic pre-treatments: None (no antibiotic, blue), Amp (ampicillin, red), Strep (streptomycin, green), and Sulf (sulfamethazine, purple), with statistical significance denoted by asterisks (* p < 0.05, **** p < 0.0001). (B) Principal coordinate analysis (PCoA) based on the Bray–Curtis dissimilarity of the gut microbiomes on 0 DPI, with colors indicating different antibiotic treatments. (C) Bar graph showing the mean relative abundance of microbial phyla from −7 to 7 DPI across different treatment groups. (D) Dot plot illustrating the differential enrichment of various genera on 0 DPI, with dot size representing the negative logarithm of the p-value and color indicating the antibiotic treatment group. (E,F) Relative abundance of Enterobacteriaceae on 0 (E) and 1 (F) DPI, with bars representing the mean values and error bars indicating standard error (SE). Statistical significance in panels (E,F) is indicated by asterisks above the bars (* p < 0.05, ** p < 0.01, **** p < 0.0001).
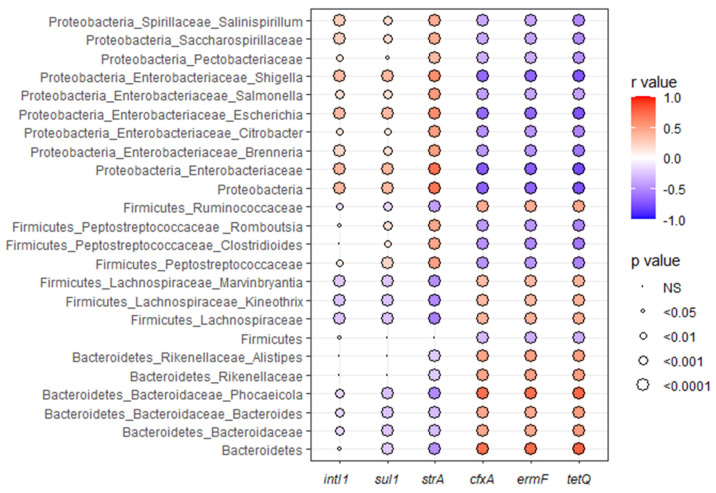
The differential-abundance analysis showed that various genera were enriched following the different antibiotic pre-treatments. After the ampicillin pre-treatment, genera such as Alistipes, Bacteroides, Enterobacter, Lachnociostridium, and Klebsiella showed enrichment. The control mice with no antibiotic pre-treatment exhibited an increase in Enterocloster, Lacrimispora, Peptococcus, and Roseburia. The streptomycin pre-treatment resulted in an enrichment of Clostridium, Escherichia, Phocaeicola, Rhodoligotrophos, Romboutsia, and Shigella. The sulfamethazine pre-treatment led to an increase in Anaerotignum, Faecalimonas, Ruminoccoccus, and Pseudoflavonifractor (Figure 4D). Among these enriched genera, Alistipes, Bacteroides, and Phocaeicola were found to be positively associated with the cfxA, ermF, and tetQ genes, while Escherichia, Romboutsia and Shigella were negatively associated with these genes (Figure 5). In addition, the Bacteroidetes phylum was positively associated with the cfxA, ermF, and tetQ genes, and the Proteobacteria phylum was negatively associated with these genes. The Proteobacteria phylum, along with the Enterobacteriaceae family and the Escherichia and Shigella genera, were positively associated with the strA, sul1, and intI1 genes (Figure 5). Within the Firmicutes phylum, the Lachnospiraceae family was associated positively with the cfxA, ermF, and tetQ genes and negatively with the strA gene, whereas the Peptostreptococcaceae family showed the opposite trend.
Figure 5.
Correlogram showing Pearson’s correlations (r values) between the abundance of an integrase gene (intI1) and specific antimicrobial resistance genes (sul1, strA, cfxA, ermF, and tetQ), and various bacterial taxa at different taxonomic levels (phylum, family, and genus) in the fecal samples of all the mice in this study. The color intensity of each dot corresponds to the strength and direction of the correlation, with red indication a positive correlation and blue a negative correlation. The size of the circles represents the level of statistical significance, with larger circles indicating a lower p-value. Non-significant correlations are marked as ‘NS’.
4. Discussion
Antibiotics are powerful medications that treat bacterial infections, but they also drive the dissemination of ARGs within a gut microbiome. The presence of antibiotics grants a selective advantage for ARB to colonize and replicate in the gut, enabling the conjugative transfer of resistance plasmids from the ARB to opportunistic pathogens and commensals, and subsequently encouraging transconjugant propagation [17,18]. Furthermore, antibiotics collaterally target commensals, altering gut microbiome composition and causing dysbiosis [42]. Immediately following an antibiotic treatment, the reduced microbial diversity favors the establishment of opportunistic pathogens such as Salmonella including antibiotic resistant Salmonella within the gut microbiota [21,43,44]. Previous studies have demonstrated the conjugative transfer of antibiotic resistance plasmids following heavy-dose streptomycin treatments for clearing the mouse gut [19,20]. Depending on the chemical composition, formulation, and administration route, antibiotics may cause different magnitudes of perturbation in gut microbiomes [45]. Despite reports on the plasmid transfers related to clinical antibiotic treatments [46,47], a deeper understanding of how microbiome disturbances facilitate the conjugative transfer of antibiotic resistance plasmids is needed.
In this study, pre-treatments with a heavy dose of streptomycin, a clinical dosage of ampicillin, and a veterinary dosage of sulfamethazine were used to generate different levels of microbiome perturbation, along with a no antibiotic group serving as the negative control. All three antibiotic pre-treatments significantly reduced the species richness, a key diversity index, with streptomycin causing the greatest reduction, followed by ampicillin, and sulfamethazine causing the least. The density of colonization by both the Salmonella donor and recipient was positively associated with the reduction in microbial diversity induced by the antibiotic pre-treatments, with streptomycin leading to the highest colonization density, followed by ampicillin, and sulfamethazine resulting in the lowest. This high-density colonization increased the potential for a conjugative transfer of the multi-drug resistance IncA/C plasmid from the Salmonella donor to recipient. Additionally, the severe Salmonella infection and gut dysbiosis caused by the streptomycin pre-treatment led to a significantly lower survival rate in the mice. In contrast, the ampicillin pre-treatment induced intermediate gut dysbiosis, supporting the colonization of the Salmonella donor but not the Salmonella recipient, thereby preventing conjugation between the two. However, both streptomycin and ampicillin pre-treatments enriched the Enterobacteriaceae family. Within this family the Escherichia and Shigella genera were significantly enriched by the streptomycin pre-treatment, and the Enterobacter and Klebsiella genera by the ampicillin pre-treatment. Although the relative abundance of Escherichia was lower following the pre-treatment with ampicillin than with streptomycin, E. coli served as a commensal recipient and supported the dissemination of the IncA/C plasmid in the gut microbiome that was disturbed by either pre-treatment. In support of our findings, Stecher et al. [48] reported that pathogen-driven inflammatory responses generated a transient expansion of the Enterobacteriaceae which promoted a horizontal gene transfer via a conjugation between the pathogens and commensals in a mouse’s gut. In comparison, the sulfamethazine pre-treatment only caused a mild microbiome perturbation with the least reduction in species richness, which did not favor the colonization of the Salmonella donor or recipient, or the conjugative transfer of the IncA/C plasmid. This finding aligns with the report by Liu et al. [49] on the negligible impact of sulfamethoxazole on the abundances of Bacteroidetes and Firmicutes in a mouse’s gut. In our control mice receiving no antibiotic pre-treatment, the normal gut microbiome resisted Salmonella colonization and eliminated the IncA/C transfer. Overall, our data suggest that the colonization of opportunistic antimicrobial-resistance food- or water-borne pathogens and the subsequent dissemination of the introduced ARGs are positively associated with the perturbation magnitude of the gut microbiome and the expansion of the Enterobacteriaceae.
Furthermore, various antibiotics may induce differential changes to the structure of a gut microbiome and drive the dissemination of ARGs in the enriched taxa. Using a C57BL/6J mouse model, de Nies et al. [13] reported that the relative abundance of ARGs, including those from the β-lactam, glycopeptide, and aminoglycoside categories, was significantly increased in the enriched Akkermansiaceae family in a gut microbiome after a treatment with an antibiotic cocktail of ampicillin, vancomycin, neomycin, and metronidazole. They suggested that the integrons associated with ARGs played a key role in mediating the AMR spread. Similarly, Xu et al. [50] observed that a mono-antibiotic treatment with ampicillin, ciprofloxacin, or fosfomycin, led to an increased relative abundance of specific bacterial species and ARGs in Balb/c mice. They suggested that the enrichment of transposases after a treatment with ciprofloxacin could signify an increased potential for horizontal gene transfer in gut microbiomes. Furthermore, a study involving human participants by Anthony et al. [42] reported a significantly increased ARG burden after treatments with cefpodoxime, azithromycin, or a combination of both, indicating antibiotic-specific changes in the ARG relative abundance. Despite these insights, a common finding across all these studies [13,42,50] was a significant reduction in species richness and alterations in the relative abundance of numerous ARGs after antibiotic treatments. In accordance with these studies, we observed that the species richness of the gut microbiomes was significantly reduced following each of the three antibiotic pre-treatments, accompanied by the significant enrichment of specific genera depending on the antibiotic used. We found a significant enrichment of spore-forming bacteria and members of the Bacteroidaceae, Rikenellaceae, and Enterobacteriaceae families. Likely, these bacteria can tolerate antibiotics through spore-mediated persistence, intrinsic resistance, and/or other resiliency mechanisms [45]. In our study, the commensal E. coli carried a mdfA gene in its chromosome. The mdfA is a multi-drug transporter gene conferring resistance to certain antibiotics such as chloramphenicol, erythromycin, and certain aminoglycosides and fluoroquinolones [51]. Similarly, we isolated a commensal Enterobacter strain carrying a blaACT gene in its chromosome. These intrinsic resistance determinants likely contributed to the enrichment of Escherichia and Enterobacter following the streptomycin and ampicillin pre-treatments, respectively. Positively associated with the Enterobacteriaceae enrichment, the abundance of the strA and sul1 genes increased after the colonization of the Salmonella donor carrying the IncA/C plasmid encoding these two genes. Clearly, the dissemination of the strA and sul1 genes in the enriched Enterbobacteriaceae via the conjugative transfer of the plasmid was affected by the magnitude of dysbiosis that was induced by the different antibiotic pre-treatments. The dynamics of the intl1 gene were similar to that of the strA and sul1 genes, influenced by the magnitude of gut dysbiosis. In support of our findings, Xu et al. [50] observed only a slight, short increase in the relative abundance of integrases in mouse gut microbiomes after an ampicillin treatment. In contrast to the introduced ARGs, the abundance of the cfxA, tetQ, and ermF genes was reduced in the disturbed gut microbiome. The reduction varied with the extent corresponding to the level of dysbiosis induced by the antibiotics, with the greatest decrease following the streptomycin pre-treatment, a lesser decrease after the ampicillin pre-treatment, and the smallest to no decrease post-sulfamethazine treatment. As the cfxA, tetQ, and ermF genes are highly prevalent in the Bacteroidetes and Firmicutes phyla [22,23], the depletion of these core commensal bacteria by the antibiotic treatments alongside the Salmonella infection likely led to the reduction of these ARGs. Overall, our findings suggest that the dynamics of ARGs are closely connected to and largely affected by the taxonomic changes in a gut microbiome.
This study aimed to evaluate the effects on the conjugative transfer of ARGs caused by varying levels of gut microbiome disturbance induced by different antibiotic pre-treatments. The varying antibiotic dosages facilitated a qualitative evaluation; however, gradient dosages of a single antibiotic might be more appropriate for a quantitative assessment. The use of conventional selective bacterial culture methods provided for the effective isolation and enumeration of S. Typhimurium transconjugants. However, these techniques had limitations when attempting to identify and recover unknown transconjugants among the commensal bacteria. Due to resource constraints, our study only focused on recovering Enterobacteriaceae transconjugants and was not able to capture any potential ARG disseminations in other taxa. To investigate the dynamics of ARGs, we used qPCRs assays targeting specific ARGs known to be associated with mobile genetic elements or inherently present in core commensal bacteria. Such an approach clearly captured the interactions between the dynamics of target ARGs and the perturbations of a gut microbiome, but was not able to cover the broader resistome changes.
5. Conclusions
This study explored the impacts of gut dysbiosis induced by various antibiotic pre-treatments on the conjugative transfer and dynamics of ARGs using a mouse model. We found a positive correlation between the potential for a conjugative transfer of a multi-drug resistance plasmid, from the S. Heidelberg donor to the S. Typhimurium recipient and the commensal E. coli, and the degree of gut dysbiosis. Furthermore, the magnitude of gut dysbiosis also affected the dynamics of the ARGs. An increase in the abundance of the sul1 and strA genes carried by the multi-drug-resistant plasmid was positively associated with the enrichment of Proteobacteria and the Enterobacteriaceae, whereas a reduction in the abundance of the cfxA, tetQ, and ermF genes was positively associated with a depletion in Bacteroidetes and Firmicutes. Our findings underline the importance of pre-existing gut dysbiosis induced by specific antibiotics on the horizontal transfer of ARGs from food- or water-borne ARB to commensal bacteria, and may help guide antibiotic treatment choices to minimize the dissemination of AMR in the gut microbiome.
Acknowledgments
Great appreciation is expressed to R. Reid-Smith at the Public Health Agency of Canada, in Guelph, Canada for providing the Salmonella Heidelberg isolate; to the staff members at the Ottawa Laboratory (Fallowfield) of the Canadian Food Inspection Agency, in Ottawa, Canada, including Sandra McIntosh, Sarah Sauvé, Krystal Belanger, and Tessia Berry for their technical assistance in caring for and handling the animals; to Hanhong Dan for his technical assistance in generating DNA standards for the qPCR assays; and to Sally Lloy and Danielle Wolfe for their technical assistance in performing the qPCR assays.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms12112148/s1: Figure S1: Enumeration of Salmonella Typhimurium and S. Heidelberg in the fecal samples; Figure S2: Survival curves of the C57BL/6 mice that were inoculated with Salmonella Typhimurium and Salmonella Heidelberg; Figure S3: Pearson correlation between the quantity of sul1, intI1, and strA genes and the number of Salmonella; Figure S4: Microbial species richness as assessed by the Chao1 index; Figure S5: Principal coordinate analysis on Bray–Curtis dissimilarity of mouse gut microbiota; Table S1: PCR primers and conditions for targeted gene quantification; Table S2: Isolates of transconjugant from the mice subjected to antibiotic pre-treatments followed by a Salmonella infection; and Table S3: Weight changes and symptoms of the mice subjected to antibiotic pre-treatments followed by a Salmonella infection.
Author Contributions
J.G. and E.T. designed the experiments. J.G. wrote the manuscript, all co-authors edited and contributed to the revisions. G.Y. and J.G. carried out the animal experiments and bacterial enumeration and analyzed the bacterial culture data. G.Y. and M.C. performed PCR for quantitation of the resistance genes. C.H.-F.L., S.C. and M.K. conducted the 16S rRNA gene amplicon sequencing analysis. E.H., P.C. and M.K. performed the whole genome sequencing analysis. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol (protocol code ACC #19-03), was approved by the Animal Care Committee at the Ottawa Laboratory (Fallowfield) of the Canadian Food Inspection Agency on 28 February 2019, in Ottawa, Canada.
Data Availability Statement
All supporting data have been provided within the article or through supplementary data files. One supplementary table and five supplementary figures are available with the online version of this article. The whole genome sequencing data generated for this study can be found in the NCBI BioProject PRJNA1079257.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was funded by the Government of Canada’s Genomics R&D Initiative Phase VI Shared Priority Project Management Plan on Antimicrobial Resistance (GRDI-AMR).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Baker S., Thomson N., Weill F.-X., Holt K.E. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. Science. 2017;360:733–738. doi: 10.1126/science.aar3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau C.H.-F., van Engelen K., Gordon S., Renaud J., Topp E. Novel antibiotic resistance determinants from agricultural soil exposed to antibiotics widely used in human medicine and animal farming. Appl. Environ. Microbiol. 2017;83:e00989-17. doi: 10.1128/AEM.00989-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson D.G.J., Andremont A., Bengtsson-Palme J., Brandt K.K., de Roda Husman A.M., Fagerstedt P., Fick J., Flach C.-F., Gaze W.H., Kuroda M., et al. Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ. Int. 2018;117:132–138. doi: 10.1016/j.envint.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 4.Smalla K., Simonet P., Tiedje J., Topp E. Editorial: Special section of FEMS Microbiology Ecology on the environmental dimension of antibiotic resistance. FEMS Microbiol. Ecol. 2016;92:fiw172. doi: 10.1093/femsec/fiw172. [DOI] [PubMed] [Google Scholar]
- 5.Berendonk T., Manaia C., Merlin C., Fatta-Kassinos D., Cytryn E., Walsh F., Burgmann H., Sorum H., Norstrom M., Pons M.-N., et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015;13:310–317. doi: 10.1038/nrmicro3439. [DOI] [PubMed] [Google Scholar]
- 6.McEwen S.A., Collignon P.J. Antimicrobial resistance: A one health perspective. Microbiol. Spectr. 2018;6:ARBA-0009-2017. doi: 10.1128/microbiolspec.ARBA-0009-2017. [DOI] [PubMed] [Google Scholar]
- 7.Food and Agriculture Organization of the United Nations The FAO Action Plan on Antimicrobial Resistance 2021–2025. 2021. [(accessed on 4 October 2024)]. Available online: https://www.fao.org/3/cb5545en/cb5545en.pdf.
- 8.Levy M., Blacher E., Elinav E. Microbiome, metabolites, and host immunity. Curr. Opin. Microbiol. 2017;35:8–15. doi: 10.1016/j.mib.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Ho J., Yeoh Y.K., Barua N., Chen Z., Lui G., Wong S.H., Yang X., Chan M.C., Chan P.K., Hawkey P.M., et al. Systematic review of human gut resistome studies revealed variable definitions and approaches. Gut Microbes. 2020;12:1700755. doi: 10.1080/19490976.2019.1700755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan R., Jin M., Shao Y., Yin J., Li H., Chen T., Shi D., Zhou S., Li J., Yang D. High-sugar, high-fat, and high-protein diets promote antibiotic resistance gene spreading in the mouse intestinal microbiota. Gut Microbes. 2022;14:2022442. doi: 10.1080/19490976.2021.2022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro C.F.A., Silveira G.G.O.S., Cândido E.S., Cardoso M.H., Espínola Carvalho C.M., Franco O.L. Effects of antibiotic treatment on gut microbiota and how to overcome its negative impacts on human health. ACS Infect. Dis. 2020;6:2544–2559. doi: 10.1021/acsinfecdis.0c00036. [DOI] [PubMed] [Google Scholar]
- 12.Ruppé E., Ghozlane A., Tap J., Pons N., Alvarez A.-S., Mazierset N., Cuesta T., Hernando-Amado S., Clares I., Martinez J.L., et al. Prediction of the intestinal resistome by a three-dimensional structure-based method. Nat. Microbiol. 2019;4:112–123. doi: 10.1038/s41564-018-0292-6. [DOI] [PubMed] [Google Scholar]
- 13.de Nies L., Busi S.B., Tsenkova M., Halder R., Letellier E., Wilmes P. Evolution of the murine gut resistome following broad-spectrum antibiotic treatment. Nat. Commun. 2022;13:2296. doi: 10.1038/s41467-022-29919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier L., Goemans C.V., Wirbel J., Kuhn M., Eberl C., Pruteanu M., Muller P., Garcia-Santamarina S., Cacace E., Zhang B., et al. Unravelling the collateral damage of antibiotics on gut bacteria. Nature. 2021;599:120–124. doi: 10.1038/s41586-021-03986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huddleston J.R. Horizontal gene transfer in the human gastrointestinal tract: Potential spread of antibiotic resistance genes. Infect. Drug. Resist. 2014;7:167–176. doi: 10.2147/IDR.S48820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopatkin A., Huang S., Smith R., Srimani J.K., Sysoeva T.A., Bewick S., Karig D., You L. Antibiotics as a selective driver for conjugation dynamics. Nat. Microbiol. 2016;1:1–22. doi: 10.1038/nmicrobiol.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye L., Chan E.W.C., Chen S. Selective and suppressive effects of antibiotics on donor and recipient bacterial strains in gut microbiota determine transmission efficiency of blaNDM-1-bearing plasmids. J. Antimicrob. Chemother. 2019;74:1867–1875. doi: 10.1093/jac/dkz137. [DOI] [PubMed] [Google Scholar]
- 18.Laskey A., Ottenbrite M., Devenish J., Kang M., Savic M., Nadin-Davis S., Chmara J., Lin M., Robertson J., Bessonov K., et al. Mobility of β-lactam resistance under bacterial co-infection and ampicillin treatment in a mouse model. Front. Microbiol. 2020;11:1591. doi: 10.3389/fmicb.2020.01591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laskey A., Devenish J., Kang M., Savic M., Chmara J., Dan H., Lin M., Robertson J., Bessono K., Gurnik S., et al. Mobility of β-lactam resistance under ampicillin treatment in gut microbiota suffering from pre-disturbance. Microb. Genom. 2021;7:000713. doi: 10.1099/mgen.0.000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aviv G., Rahav G., Gal-Mor O. Horizontal transfer of the Salmonella enterica serovar Infantis resistance and virulence plasmid pESI to the intestine microbiota of warm blooded hosts. mBio. 2016;7:e01395-16. doi: 10.1128/mBio.01395-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mei X., Ma B., Zhai X., Zhang A., Lei C., Zuo L., Yang X., Zhou C., Wang H. Florfenicol enhances colonization of a Salmonella enterica Serovar Enteritidis floR mutant with major alterations to the intestinal microbiota and metabolome in neonatal chickens. Appl. Environ. Microbiol. 2021;87:e0168121. doi: 10.1128/AEM.01681-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamberte L.E., van Schaik W. Antibiotic resistance in the commensal human gut microbiota. Curr. Opin. Microbiol. 2022;68:102150. doi: 10.1016/j.mib.2022.102150. [DOI] [PubMed] [Google Scholar]
- 23.Wu L., Xie X., Li Y., Liang T., Zhong H., Ma J., Yang L., Yang J., Li L., Xi Y., et al. Metagenomics-based analysis of the age-related cumulative effect of antibiotic resistance genes in gut microbiota. Antibiotics. 2021;10:1006. doi: 10.3390/antibiotics10081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souvorov A., Agarwala R., Lipman D. SKESA: Strategic k-mer extension for scrupulous assemblies. Genome Biol. 2018;19:153. doi: 10.1186/s13059-018-1540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurevich A., Saveliev V., Vyahhi N., Tesler G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson J., Nash J.H.E. MOB-suite: Software tools for clustering, reconstruction and typing of plasmids from draft assemblies. Microb. Genom. 2018;4:e000206. doi: 10.1099/mgen.0.000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next generation sequencing based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S., Zhou Y., Chen Y., Gu J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X., Shao Y., Tian J., Liao Y., Li P., Zhang Y., Chen J., Li Z. pTrimmer: An efficient tool to trim primers of multiplex deep sequencing data. BMC Bioinform. 2019;20:236. doi: 10.1186/s12859-019-2854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curry K.D., Wang Q., Nute M.G., Reeves E., Soriano S., Wu Q., Graeber E., Finzer P., Mendling W., Savidge T., et al. Emu: Species-level microbial community profiling of full-length 16S rRNA Oxford Nanopore sequencing data. Nat. Methods. 2022;19:845–853. doi: 10.1038/s41592-022-01520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aminov R.I., Garrigues-Jeanjean N., Mackie R.I. Molecular ecology of tetracycline resistance: Development and validation of primers for detection of tetracycline resistance genes encoding ribosomal protection proteins. Appl. Environ. Microbiol. 2001;67:22–32. doi: 10.1128/AEM.67.1.22-32.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyer T.C., Singer R.S. Quantitative measurement of blaCMY-2 in a longitudinal observational study of dairy cattle treated with ceftiofur. Foodborne Pathog. Dis. 2012;9:1022–1027. doi: 10.1089/fpd.2012.1198. [DOI] [PubMed] [Google Scholar]
- 33.Eitel Z., Sóki J., Urbán E., Nagy E. The prevalence of antibiotic resistance genes in Bacteroides fragilis group strains isolated in different European countries. Anaerobe. 2013;21:43–49. doi: 10.1016/j.anaerobe.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Gaze W.H., Zhang L., Abdouslam N.A., Hawkey P.M., Calvo-Bado L., Royle J., Brown H., Davis S., Kay P., Boxall A.B.A., et al. Impacts of anthropogenic activity on the ecology of class 1 integrons and integron-associated genes in the environment. ISME J. 2011;5:1253–1261. doi: 10.1038/ismej.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knapp C.W., Zhang W., Sturm B.S.M., Graham D.W. Differential fate of erythromycin and beta-lactam resistance genes from swine lagoon waste under different aquatic conditions. Environ. Pollut. 2010;158:1506–1512. doi: 10.1016/j.envpol.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Malorny B., Bunge C., Helmuth R. Evaluation of Salmonella spp. Specific Primer-Sets for the Validation Within the Food PCR Project. 2001. [(accessed on 16 October 2019)]. Available online: https://mobil.bfr.bund.de/cm/343/evaluation_of_salmonella_spp._specific_primer_sets_for_the_validation.pdf.
- 37.Marti R., Tien Y.C., Murray R., Scott A., Sabourin L., Topp E. Safely coupling livestock and crop production systems: How rapidly do antibiotic resistance genes dissipate in soil following a commercial application of swine or dairy manure? Appl. Environ. Microbiol. 2014;80:3258–3265. doi: 10.1128/AEM.00231-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resende J.A., Diniz C.G., Silva V.L., Otenio M.H., Bonnafous A., Arcuri P.B., Godon J.-J. Dynamics of antibiotic resistance genes and presence of putative pathogens during ambient temperature anaerobic digestion. J. Appl. Microbiol. 2014;117:1689–1699. doi: 10.1111/jam.12653. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki M.T., Taylor L.T., DeLong E.F. Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl. Environ. Microbiol. 2000;66:4605–4614. doi: 10.1128/AEM.66.11.4605-4614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh F., Ingenfeld A., Zampicolli M., Hilber-Bodmer M., Frey J.E., Duffy B. Real-time PCR methods for quantitative monitoring of streptomycin and tetracycline resistance genes in agricultural ecosystems. J. Microbiol. Methods. 2011;86:150–155. doi: 10.1016/j.mimet.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anthony W.E., Wang B., Sukhum K.V., D’Souza A.W., Hink T., Cass C., Seiler S., Reske K.A., Coon C., Dubberke E.R., et al. Acute and persistent effects of commonly used antibiotics on the gut microbiome and resistome in healthy adults. Cell Rep. 2022;39:110649. doi: 10.1016/j.celrep.2022.110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scanlan P.D. Microbial evolution and ecological opportunity in the gut environment. Proc. Biol. Sci. 2019;286:20191964. doi: 10.1098/rspb.2019.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers A.W.L., Tsolis R.M., Bäumler A.J. Salmonella versus the microbiome. Microbiol. Mol. Biol. Rev. 2021;85:e00027-19. doi: 10.1128/MMBR.00027-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fishbein S.R.S., Mahmud B., Dantas G. Antibiotic perturbations to the gut microbiome. Nat. Rev. Microbiol. 2023;21:772–788. doi: 10.1038/s41579-023-00933-y. [DOI] [PubMed] [Google Scholar]
- 46.Conlan S., Lau A.F., Deming C., Spalding C.D., Lee-Lin S., Thomas P.J., Park M., Dekker J., Frank K., Palmore T., et al. Plasmid dissemination and selection of a multidrug resistant Klebsiella pneumoniae strain during transplant-associated antibiotic therapy. mBio. 2019;10:e00652-19. doi: 10.1128/mBio.00652-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gude M.J., Seral C., Sáenz Y., Cebollada R., González-Domínguez M., Torres C., Castillo F.J. Molecular epidemiology, resistance profiles and clinical features in clinical plasmid-mediated AmpC-producing Enterobacteriaceae. Int. J. Med. Microbiol. 2013;303:553–557. doi: 10.1016/j.ijmm.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Stecher B., Denzler R., Maier L., Bernet F., Sanders M.J., Pickard D.J., Barthel M., Westendorf A., Krogfelt K., Walker A., et al. Intestine inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc. Natl. Acad. Sci. USA. 2012;109:1269–1274. doi: 10.1073/pnas.1113246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J., Lv M., Sun A., Ding J., Wang Y., Chang X., Chen L. Exposure to microplastics reduces the bioaccumulation of sulfamethoxazole but enhances its effects on gut microbiota and the antibiotic resistome of mice. Chemosphere. 2022;294:133810. doi: 10.1016/j.chemosphere.2022.133810. [DOI] [PubMed] [Google Scholar]
- 50.Xu L., Surathu A., Raplee I., Chockalingam A., Stewart S., Walke L., Sacks L., Patel V., Li Z., Rouse R. The effect of antibiotics on the gut microbiome: A metagenomics analysis of microbial shift and gut antibiotic resistance in antibiotic treated mice. BMC Genom. 2020;21:263. doi: 10.1186/s12864-020-6665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edgar R., Bibi E. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J. Bacteriol. 1997;179:2274–2280. doi: 10.1128/jb.179.7.2274-2280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting data have been provided within the article or through supplementary data files. One supplementary table and five supplementary figures are available with the online version of this article. The whole genome sequencing data generated for this study can be found in the NCBI BioProject PRJNA1079257.