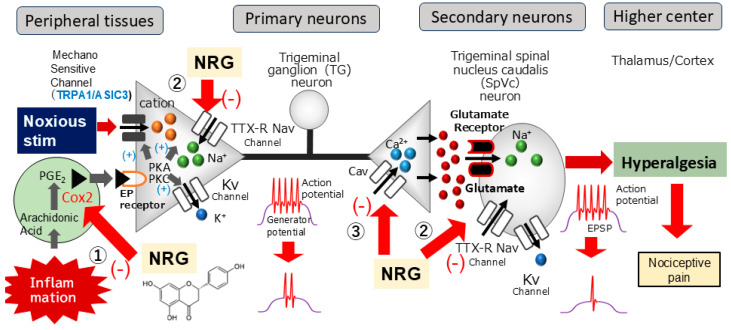
Figure 5.
A possible mechanism underlying NRG suppression of inflammation-induced mechanical hyperalgesia. Following peripheral inflammation, inflammatory mediators, such as prostaglandins (PGE2), attach to G protein-coupled E prostanoid (EP) receptors, triggering the activation of protein kinase A and C (PKA and PKC, respectively) in pain-detecting peripheral nerve endings, leading to phosphorylation of mechanosensitive transient receptor potential/acid-sensing ion channels (TRP/ASIC), Na+ (Nav), and K+ (Kv) channels and receptors. As a result, the activation threshold for transducer channels, such as the TRP channel family, including TRPA1, is diminished, leading to an increase in membrane excitability at the peripheral terminals, which causes a higher frequency of action potentials being conducted to presynaptic central terminals of the SpVc. Ultimately, more glutamate is released into the synaptic cleft and binds to the increased number of post-synaptic glutamate receptors, augmenting excitatory post-synaptic potentials (EPSPs) and triggering a flow of action potentials transmitted to higher pain pathway centers, producing an intensified sensitivity referred to as peripheral sensitization. There is a possibility that systemic delivery of NRG reduces hyperalgesia caused by inflammation-induced mechanical hyperalgesia, with this effect primarily due to suppression of the hyperexcitability of SpVc WDR neurons via inhibition of the peripheral cyclooxygenase (Cox)-2 cascade signaling pathways (①), tetrodotoxin-resistant (TTX-R) Nav channels (②), and the central terminal of Cav channels (③), decreasing the firing frequency of action potentials in the nociceptive nerve terminals and inhibiting the conduction of pain signals to the SpVc and higher centers for lateral and medial pain control (hyperalgesia).