Abstract
Background/Objectives: Population aging is a worldwide phenomenon and is often associated with multimorbidity and polypharmacy. Complex medication regimens are common among older adults and contribute to the occurrence of harmful health outcomes. Age is one of the main risk factors for cancer. This study aimed to determine and characterize the therapeutic complexity in older patients with cancer, and analyze the factors associated with high complexity and the impact of the oncological context. Methods: A cross-sectional study with patients aged ≥65 years with cancer was conducted in three hospitals in northern Portugal. Data collection was obtained using self-reports. The medication regimen complexity was assessed using the Medication Regimen Complexity Index (MRCI). Descriptive and association statistical analysis were performed. Logistic, linear, simple and multiple regression analysis were conducted, with and without automatic variable selection. Results: A total of 552 patients were included (median age, 71; IQR, 68–76). The mean MRCI before the oncological context was 18.67 (SD 12.60) and 27.39 (SD 16.67) after the oncological context, presenting a statistically significant difference in the values obtained (p < 0.001). An elevated complexity was significantly associated with polypharmacy, chronic diseases and with the administration of high-risk medications (p < 0.05). High MRCI values showed a relationship with the occurrence of potential drug interactions (p < 0.001). There was no relationship with the existence of cardiac risk comorbidity. Conclusions: This study demonstrated the existence of high therapeutic complexity in older patients with cancer, suggesting the need for intervention to prevent medication-related problems in this vulnerable population.
Keywords: Medication Regimen Complexity Index, MRCI, older adults, aging, cancer, polypharmacy
1. Introduction
Population aging is a global phenomenon. According to the World Health Organization (WHO), the number of older persons (≥65 years) is expected to reach 1.5 billion by 2050, representing approximately 16% of the population worldwide [1].
The high prevalence of chronic diseases in older adults [2] predisposes this population to the occurrence of polypharmacy contexts (use of five or more medications) and they have more complex medication regimens [3,4,5,6,7]. Due to the frequent presence of reduced manual dexterity and cognitive and sensory impairment they may face greater difficulty in managing their medication, making them more vulnerable to medication errors and medication-related problems [8,9].
Age is one of the main cancer risk factors due to biological changes associated with the aging process [10,11,12,13,14]. In the last few years there has been an increase in cancer incidence in many countries, which is primarily ascribed to a significant increase in the senior population. It is predicted that by 2040, 47% of all new cancer diagnoses will be in adults aged ≥70 years [11]. Older adults with cancer have a high comorbidities burden and polypharmacy is also common [15]. These patients have an additional medication burden because, in addition to cancer treatment, they are often administered medications to treat chronic diseases and supplementary supportive care medications. In being more vulnerable to adverse drug effects, geriatric patients with cancer undergoing chemotherapy tend to be more exposed to the risks of drug-related problems [16].
Therapeutic complexity may arise as a result of the number of medications, but other factors such as the administration of different dosage forms, multiple daily dosages and additional administration instructions must also be considered [8,9,17,18,19,20,21]. Medication complexity has been linked to negative health outcomes [22,23,24] such as non-adherence to medication [24,25,26], adverse drug reactions, drug interactions, hospitalizations [24,27], increased use of resources, increased cost of healthcare, decreased quality of life [28] and a decline in functional status and mortality [28,29,30]. In order to reduce the negative aspects identified, it is imperative to thrive in the simplification of medication regimens. Interventions that can reduce the complexity of medication and improve the patient’s quality of life and functional status are of great importance for older adults with cancer.
Different methods have been used to quantify the complexity of medication regimens. The Medication Regimen Complexity Index (MRCI), developed by George et al. [31], is the most used, reliable and validated tool for this purpose, having already been translated and validated into several languages [32,33,34,35,36] and applied in different contexts [37,38,39,40]. The MRCI is a tool that quantifies medication regimen complexity beyond the number of medications to include weighted scores for the types of dosage forms prescribed, dosing frequency and additional administration instructions, for each medication administered [31,41,42]. The MRCI allows a comprehensive and complete assessment and evaluation of a patient’s medication therapy regimen, allowing the identification of patients for intervention who require medication management [42,43]. The medication review has been shown to be an essential service to ensure medication safety in older patients with cancer, by preventing medication errors, identifying drug–drug interactions, adjusting chemotherapy doses and initiating deprescribing [44].
Complex medication regimens and the existence of polypharmacy make these patients more susceptible to the occurrence of medication errors and drug interactions, with particularly serious consequences in patients taking high-risk medications (e.g., warfarin, opioids, insulin) [38,45,46]. Likewise, careful attention should be given to patients with cardiovascular comorbidities and/or diabetes, which are prone to decompensate during anticancer treatment and often have been prescribed multiple drugs [21,43,44,47].
The present study aimed to determine and characterize therapeutic complexity in older patients diagnosed with cancer, and analyze the factors associated with high complexity and the impact of the oncological context on the complexity of the registered therapeutic regimen. This study also aimed to relate the MRCI to the existence of polypharmacy, potential drug interactions, administration of high-risk medications and the existence of comorbidities with cardiac risk.
2. Results
A total of 552 patients were included in this study, of which 308 were male (55.69%). The median age was 71 years (Interquartile Range (IQR), 68–76), with 8.88% of the patients being older than 80 years. The mean age was 71.88 years (SD 5.04). Regarding chronic diseases, 88.41% of the sample (N = 488) had at least one chronic disease and 60.14% (N = 332) presented more than two. Other common non-cancer diagnoses included hypertension (53.99%), dyslipidemia (38.95%) and diabetes mellitus (22.64%), in which 66.49% of the patients had at least one of the diseases (N = 367). The most common cancer types were “digestive system tumors” (36.23%), “lung, pleural, and thymic tumors” and “breast tumors” (both with 15.94%). The baseline characteristics of the sample are summarized in Table 1.
Table 1.
Baseline characteristics of enrolled patients (N = 552).
| Variable | n | % |
|---|---|---|
| Age, median (IQR), years | 71 | (68–76) |
| Age, mean (SD) | 71.88 | (5.04) |
| 65–79 | 503 | 91.12% |
| >80 | 49 | 8.88% |
| Sex | ||
| Male | 308 | 55.69% |
| Female | 244 | 44.31% |
| Comorbidities | ||
| No | 64 | 11.59% |
| Yes | 488 | 88.41% |
| ≥2 | 332 | 60.14% |
| Comorbidities/Diseases | ||
| Heart | 353 | 63.95% |
| Endocrine | 143 | 25.91% |
| Osteoarticular | 109 | 19.75% |
| Visual | 85 | 15.40% |
| Digestive | 78 | 14.13% |
| Respiratory | 65 | 11.78% |
| Neurological | 56 | 10.14% |
| Other | 240 | 43.48% |
| Comorbidities with cardiac risk (at least one) | 367 | 66.49% |
| Diabetes mellitus | 125 | 22.64% |
| Hypertension | 298 | 53.99% |
| Dyslipidemia | 215 | 38.95% |
| Cancer type (ICD-10) | ||
| Malignant neoplasms of digestive organs | 200 | 36.23% |
| Malignant neoplasms of respiratory and intrathoracic organs | 88 | 15.94% |
| Malignant neoplasm of breast | 88 | 15.94% |
| Malignant neoplasms of male genital organs and urinary tract | 60 | 10.87% |
| Other | 116 | 21.01% |
Abbreviations: IQR, Interquartile Range; SD, standard deviation.
Regarding the medication administered, the prevalence of polypharmacy was 49.01% (N = 271) and a total of 266 (48.19%) patients took high-risk medications. Potential drug–drug interactions (DDIs) were identified in 76.45% of the patients (N = 422) and severe drug interactions (SDIs) were reported in 56.16% of patients (N = 310). The mean MRCI of all patients before the oncological context was 18.67 (SD 12.60), and 27.39 (SD 16.67) after the oncological context. The MRCI section with a higher mean was additional instructions followed by dosing frequency (Table 2).
Table 2.
Medications and MRCI.
| Variable | Descriptive Statistics/Frequency | |
|---|---|---|
| n | % | |
| Medications | ||
| 0–4 | 239 | 43.3% |
| ≥5 | 271 | 49.01% |
| ≥10 | 42 | 7.61% |
| High-risk medication | 266 | 48.19% |
| Patients exposed to DDIs | 422 | 76.45% |
| Patients exposed to SDIs | 310 | 56.16% |
| MRCI Initial (before the oncological context) | ||
| Mean, median (SD, IQR) | 18.67, 16.00 (12.60, 9.38–24.63) | |
| MRCI Final (after the oncological context) | ||
| Mean, median (SD, IQR) | 27.39, 23.75 (16.67, 16.00–38.00) | |
| MRCI—Sections (after the oncological context) | ||
| Section A—Dosage Form, mean (SD) | 2.07 (2.02) | |
| Section B—Dosing Frequency, mean (SD) | 6.48 (4.42) | |
| Section C—Additional Instructions, mean (SD) | 18.8 (11.7) | |
Abbreviations: DDIs, drug–drug interactions; SDIs, severe drug interactions; MRCI, Medication Regimen Complexity Index; SD, standard deviation; IQR, Interquartile Range.
Considering the patients’ oncological context, in the simple analysis the high total MRCI value was significantly associated with the existence of polypharmacy, excessive polypharmacy, chronic diseases, existence of comorbidities with cardiac risk (hypertension, dyslipidemia and diabetes mellitus) and the administration of high-risk medications (p < 0.001). There was no statistically significant relationship between total MRCI values and gender or age (p > 0.05). However, in a subsequent analysis, performing a multiple linear regression and analyzing all the variables, with and without automatic variable selection, only a significant association was observed between the total MRCI values with the existence of polypharmacy, excessive polypharmacy, chronic diseases and the administration of high-risk medications (p < 0.05) (Table 3).
Table 3.
Factors associated with the MRCI (linear regression for the MRCI outcome).
| Simple | Multiple | Best AIC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | Beta | 95% CI1 | p-Value |
| Age | 0.10 | −0.17, 0.36 | 0.476 | −0.05 | −0.18, 0.08 | 0.412 | |||
| Sex | −1.3 | −4.1, 1.6 | 0.382 | 0.41 | −1.2, 2.1 | 0.621 | |||
| Polypharmacy | 26 | 24, 28 | <0.001 | 21 | 19, 22 | <0.001 | 21 | 20, 23 | <0.001 |
| Excessive polypharmacy | 39 | 35, 43 | <0.001 | 27 | 24, 29 | <0.001 | 27 | 24, 29 | <0.001 |
| Chronic diseases | 12 | 7.9, 16 | <0.001 | 3.5 | 1.2, 5.8 | 0.003 | 3.6 | 1.5, 5.7 | <0.001 |
| Hypertension | 8.3 | 5.6, 11 | <0.001 | 0.28 | −1.3, 1.8 | 0.723 | |||
| Dyslipidemia | 8.2 | 5.4, 11 | <0.001 | −0.32 | −1.9, 1.2 | 0.686 | |||
| Diabetes mellitus | 12 | 8.4, 15 | <0.001 | 1.1 | −0.63, 2.8 | 0.214 | |||
| High-risk medications | 13 | 10, 16 | <0.001 | 3.2 | 1.0, 5.3 | 0.004 | 2.9 | 1.1, 4.8 | 0.002 |
Abbreviations: CI, confidence interval; AIC, Akaike Information Criterion.
MRCI values showed a statistically significant association with the occurrence of potential DDIs and SDIs (p < 0.001) (Table 4). The higher the MRCI values, the higher the number of DDIs and SDIs.
Table 4.
Association between the MRCI and the occurrence of drug interactions (linear and logistic regression).
| DDIs (Quantitative) | SDIs (Binary) | |||||
|---|---|---|---|---|---|---|
| Characteristic | Beta | 95% CI | p-Value | OR | 95% CI | p-Value |
| MRCI Final | 0.11 | 0.09, 0.13 | <0.001 | 1.05 | 1.04, 1.07 | <0.001 |
Abbreviations: DDIs, drug–drug interactions; SDIs, severe drug interactions; MRCI, Medication Regimen Complexity Index; CI, confidence interval; OR, odds ratio.
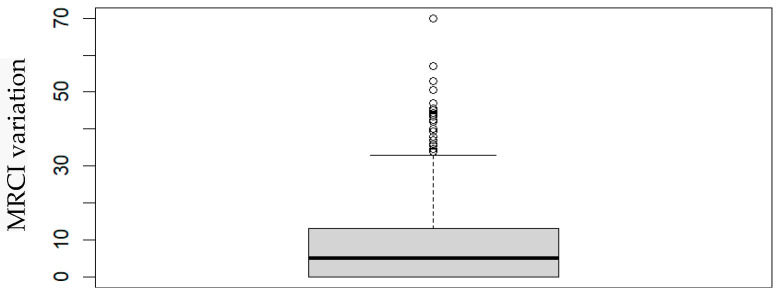
In order to analyze the impact of the oncological context on the registered MRCI, we compared the final MRCI (which includes the medication administered in the oncological context) with the initial MRCI (considering only the medication before the oncological context). Comparing the MRCI before and after the oncological context, it was possible to observe a statistically significant difference between them (sign test; p < 2.2 × 10−16), which is higher considering the patients’ oncological context (Figure 1).
Figure 1.
Boxplot of the MRCI variation at the two contexts.
Carrying out a multiple logistic regression analysis, considering all variables, with and without automatic variable selection, it was found that the observed difference can be justified by polypharmacy, excessive polypharmacy and chronic diseases (p < 0.05), which appear to have an impact on MRCI values, and is higher when considering the patients’ oncological context. The previous presence of hypertension or dyslipidemia seems to have a smaller impact on the MRCI variation in both contexts. The data presented from the multiple analysis and AIC are coherent (Table 5).
Table 5.
Factors associated with the MRCI at the two contexts (before and after the oncological context).
| Simple | Multiple | Best AIC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | Beta | 95% CI | p-Value |
| Age | −0.05 | −0.24, 0.13 | 0.562 | −0.11 | −0.26, 0.05 | 0.179 | |||
| Sex | −1.9 | −3.8, 0.06 | 0.057 | −1.7 | −3.6, 0.25 | 0.087 | −1.4 | −2.9, 0.19 | 0.085 |
| Polypharmacy | 11 | 9.8, 13 | <0.001 | 10 | 8.2, 12 | <0.001 | 10.0 | 8.3, 12 | <0.001 |
| Excessive polypharmacy | 18 | 15, 22 | <0.001 | 13 | 10, 16 | <0.001 | 13 | 10, 16 | <0.001 |
| Chronic diseases | 5.6 | 2.6, 8.6 | <0.001 | 3.7 | 1.0, 6.4 | 0.007 | 3.5 | 0.88, 6.2 | 0.009 |
| Hypertension | 1.2 | −0.79, 3.1 | 0.243 | −2.2 | −4.1, −0.37 | 0.019 | −2.3 | −4.1, −0.52 | 0.011 |
| Dyslipidemia | 1.3 | −0.65, 3.2 | 0.195 | −1.8 | −3.5, −0.02 | 0.047 | −2.0 | −3.7, −0.33 | 0.019 |
| Diabetes mellitus | 3.4 | 1.2, 5.7 | 0.003 | −0.46 | −2.4, 1.5 | 0.646 | |||
Abbreviations: CI = confidence interval; AIC, Akaike Information Criterion.
3. Discussion
Our study aimed to quantify the complexity of the medication regimen using the MRCI tool and analyze associated factors in a sample of older patients with cancer. The results demonstrate the impact of the oncological context on the MRCI values obtained, observing a statistically significant difference when comparing the MRCI before and after the beginning of oncological treatment (p < 2.2× 10−16). The variables influencing the recorded difference were the existence of polypharmacy, excessive polypharmacy and chronic diseases (p < 0.05). The results can be justified by the necessity of administering more medications, including supportive ones. These findings can also be explained by the oncological context, which often involves the use of medications with more complex instructions for the treatment of various symptoms and/or associated complications.
The complexity of the therapeutic regimen was high (mean MRCI = 27.39), with values similar to those obtained in previous studies [48,49]. Different results were found in prior research that presented lower values [9,50,51,52,53]. It is important to note that to calculate the MRCI, and specifically regarding additional instructions for medication use, some studies assume that patients administered their medications according to the standard instructions for use described in key reference texts. Our study considers the user’s report regarding the effective use of the medication, which may justify the differences recorded.
A significant association was observed between the total MRCI values and the existence of polypharmacy, excessive polypharmacy, chronic diseases and the administration of high-risk medications (p < 0.05). The results are in line with previous studies that investigated the MRCI in older patients with multiple comorbidities and polypharmacy and who, consequently, present a greater complexity of the medication regimen [4,41,53,54,55].
It was also possible to observe that high MRCI values had a statistically significant relationship with the occurrence of potential DDIs and SDIs (p < 0.001). High MRCI values can, therefore, translate into greater patient exposure to the occurrence of drug interactions, which can compromise the effectiveness of treatments and jeopardize the patients’ safety [56]. There was no isolated, statistically significant relationship between the previous existence of diseases and cardiovascular risk (hypertension, dyslipidemia and diabetes mellitus) (p > 0.05). However, studies have recorded high MRCI values in patients with these pathologies. With the increasing prevalence of these diseases in the older cancer population, and the associated high treatment costs, simplification of medication regimens in these patients may be important to achieve intended therapeutic goals [57,58].
Although polypharmacy is identified as an important risk factor for the MRCI, Wimmer et al. (2016) states that the MRCI was a better overall predictor of mortality than polypharmacy, especially in patients ≤80 years old. Patients over 80 may have other non-drug risk factors for death and, as patients with a more limited life expectancy, may have more simplified therapeutic regimens [45]. Determining the MRCI is equally important because high values can lead to increased medication errors associated with the complexity of medication use instructions. A higher complexity regimen has also been associated with non-adherence to therapy [26,41], which, in turn, is a risk factor for therapeutic ineffectiveness, compromising the expected clinical result and ongoing treatment.
In the older population, medication administration is the main cause of preventable hospitalization due to the occurrence of adverse drug events (ADEs) [59], which may occur due to the complexity of the therapeutic regimen. An example of this is the administration of high-risk medications (e.g., anticoagulants, antiplatelets, oral hypoglycemic, insulins or opioid medications) due to a potentially variable dosage, the need for injection and possible transdermal distribution. These medications are also associated with potentially fatal ADEs, such as bleeding, hypoglycemia, falls and fractures [45,50,59,60].
The results obtained in this study reinforce the importance of high-risk medications in the MRCI. For this reason, although not included independently in the MRCI tool, the administration of these medications appears to be a relevant aspect to consider in determining the MRCI.
Because they are associated with ADEs and other complications, determining the MRCI is particularly important in severe and/or more demanding clinical contexts that require more complex medication regimens, such as chronic obstructive pulmonary disease, diabetes, patients with chronic kidney disease and diseases characterized by the use of medications with complex instructions for the treatment of comorbidities and complications associated with the disease itself or the treatment implemented, as in the case of cancer patients [45,61,62].
When we compare our results with MRCI studies in other disease contexts, we see that older adults with cancer have greater medication regimen complexity than patients with heart failure, heart transplant, depression, HIV, diabetes and hypertension [43,48,53,57,63,64]. Cancer patients are among the highest-risk patients and were most likely to have highly complex regimens. Many of these patients simultaneously have other chronic diseases, which aggravates the risk of the MRCI, which can translate into negative clinical outcomes.
In future studies, it is important to evaluate the impact of therapeutic complexity on hospital readmission and hospitalization in this group of patients. Previous studies conducted in other clinical settings found that medication regimen complexity was associated with hospital admissions and readmissions [65,66]. In some of these studies, the medication regimen complexity was not identified as a better predictor of hospitalization than the number of medications [27,29,50,61,67,68]. Chang et al. (2017), in their study, suggest that hospitalization appears to be associated with increased medication complexity and the overall number of medications prescribed [52]. The two parameters are considered good indicators of older patients’ risk of hospitalization [50].
Although studies suggest that the number of medications and the MRCI have a great impact, other factors may also be related to hospital readmission and hospitalizations, such as the patient’s own characteristics, existence of comorbidities, previous hospitalizations, length of stay, complexity of the pathological context and specific characteristics of certain medication. Even so, the complexity of the therapeutic regimen has a great impact on hospital readmission, presenting an important clinical implication, as, unlike simple medication counting, this tool has different parameters that can guide during the medication review [61]. The medication review has proven to be an essential task to ensure the safety of older cancer patients, preventing medication errors, identifying drug interactions, adjusting doses and initiating deprescription [44]. Future studies may include evaluating the use of the MRCI in clinical trial protocols for new chemotherapeutic agents, in order to optimize the therapeutic regimens implemented, ensuring continuity of treatments and avoiding medication-related problems. It is equally important to use the MRCI tool across specific cancer types and stages. This may allow us to obtain more precise conclusions and guide clinical practices in more specific contexts.
The MRCI is therefore a useful tool for identifying patients who may benefit from medication therapy management intervention [42]. Metz et al. (2014) stated that the use of the MRCI provided a better perspective of which patients might be at greater risk for failing to achieve desired outcomes [64]. This tool therefore does not simply assess the number of medications a patient is taking: it also assesses points (indicating greater complexity) for the formulation (e.g., that require special devices, such as inhalers or injections, that are more complex than a tablet), dosing frequency (e.g., more times per day is more complex) and any additional directions the patient needs to follow (e.g., take at a specific time of day; take 1 h before meals; take medication on an empty stomach; and the need to divide tablets) [31,32].
Multiple formulations, several dosing frequencies and additional instructions likely complicate any patient’s ability to maintain proper and consistent medication administration practices. Case of older adults may be even more demanding due to the limitations in vision, hearing, dexterity or memory, with impaired cognition and polypharmacy [17,24,53,62]. Similar to previous studies, in our results the dosing frequency and additional instructions are the two parameters that most contributed to the overall MRCI score. These parameters should be considered by health professionals in the medication therapy management intervention.
Our results suggest that medication regimens should be reviewed for possible complexity reductions, such as removing unnecessary medications (reducing the number of medications administered) and/or simplifying dosing regimens and instructions for medication administration. Reducing dosing frequency has been identified as the intervention with the greatest potential to simplify the therapeutic regimen, particularly through the use of long-acting medications [4,27,69]. This may be of particular relevance in older patients presenting cognitive deficits and without support in managing their medication [61]. As it is not possible to safely reduce complexity, because all prescribed medications are necessary, knowledge of this is essential as it allows the identification and reduction of risks associated with the high medication regimen complexity [42].
Although determining the MRCI is not as immediate and easy to implement in routine clinical practice compared to determining the number of medications, studies show that MRCI scores can be automatically calculated and integrated into electronic health records in a way that it can assist clinical decision making [43,50,61,70]. It is therefore necessary to simplify and automatize the MRCI application to become a practical, fast, feasible and easy-to-use tool when caring for patients and managing drug therapy in primary care, in community pharmacies and in a hospital context. The MRCI can be used as a tool in clinical and pharmaceutical practice to identify patients whose therapeutic regimens are highly complex and who can benefit from an intervention. The identification of factors associated with high therapeutic complexity is useful in order to direct interventions to be implemented, and thus prevent unwanted clinical outcomes such as ADEs and hospitalizations [42,71].
Strengths and Limitations
To our knowledge, this is the first study carried out in older people in an oncological context that investigated therapeutic regimen complexity and its relationship with polypharmacy, drug interactions, the existence of chronic diseases, the presence of cardiovascular risk diseases and the administration of high-risk medications common in the geriatric population. The data were collected by trained staff and the medication regimen complexity was measured using a validated measure. It is important to acknowledge that despite the relevance of the reported data, our results may not be generalizable to other older populations due to the median age of the sample (71 years) as it is not a very aged sample in relation to average life expectancy. In addition, there may be other variables not considered, which may alter the results obtained. Furthermore, there are differences in the structure and availability of health and social services in relation to other countries. Also, although we adjusted our analyses for clinically important variables, as with all observational studies, the possibility of residual confounding cannot be excluded. Another potential limitation is that the determination of the MRCI occurs only at one point in time; however, patients receive care from multiple healthcare professionals during their treatment period, which may result in changes in medication and consequently in its complexity regimen. In other words, our analysis did not consider changes in medication regimens during the treatment period. In this study, the suitability of medications administered by patients was not analyzed. Data were collected through self-report, which may have resulted in inaccuracies and/or omissions of administered medications. Still, questioning patients about their medication use, rather than analyzing data on prescribed or dispensed medications, provided potentially more accurate information on actual medication use.
4. Materials and Methods
4.1. Study Setting, Participants and Eligibility
This is a cross-sectional study conducted at three hospitals in Porto, in northern Portugal, during a period of 16 months. It included 552 participants. Patients were flagged and invited to participate in this study by the nursing team while undergoing their chemotherapy treatment, respecting this study’s inclusion criteria: older adults with a diagnosis of cancer, aged 65 or over, with no cognitive impairment. Cognitive status was assessed using the six-item Cognitive Impairment Test (6 CIT) [72]. The exclusion criteria were not mastering the Portuguese language or not being responsible for managing one’s own medication. Data collection was carried out by the research team through direct contact with patients. Patients with incomplete data were excluded from the analysis. A non-probabilistic sampling for convenience was performed, in which the sample size was calculated using EpiInfo™® (Version 7.1.5/2015). This study was carried out after approval by the Health Ethics Committees of the three hospital institutions where this study took place and written informed consent to participate was obtained for each participant.
4.2. Data Collection
Data collection was conducted using a structured questionnaire applied to all participants. The collected data included standard demographic information (age and sex), medical conditions, identifying the type of cancer and the existence of other chronic diseases, including diseases with cardiovascular risk (diabetes mellitus, hypertension and dyslipidemia), and a detailed list of all medications administered. Information about medication use was obtained using self-reports. Information on the pharmaceutical form, therapeutic regimen and administration precautions was collected. High-risk medications were identified according to the following categories: anticoagulants, antiplatelet agents, insulin, oral hypoglycemic agents, opioids and antiarrhythmic drugs [59,73]. The oncological context of the patients was coded by the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10)—WHO (Version 2019).
4.3. Outcome Measurements
In this study, polypharmacy was defined as the use of five or more medications. The use of ten or more medications was labeled excessive polypharmacy [73,74,75,76,77]. Potential drug–drug interactions (DDIs) were assessed using the Micromedex® (electronic) [78]. The most valued and clinically relevant DDIs were severe drug interactions (SDIs), which included major and contraindicated interactions. The Micromedex® solutions database has been used in other oncology drug interaction studies [79,80].
4.4. Assessment of Medication Regimen Complexity
The medication regimen complexity was assessed using the MRCI. Originally developed and validated by George et al. (2004), the MRCI is a standardized tool, considered valid and reliable for measuring the complexity of medication regimens [31,32,42]. The MRCI is a 65-item instrument that can be used to quantify medication regimen complexity, and presents three sections: (A) dosage form (32 items) (tablet/capsule, paste, injectable, etc.); (B) dosing frequency (23 items) (once a day, twice a day, etc.); and (C) additional instructions for use (10 items) (take with food, crush/break the tablet, alternating dosage, etc.). The MRCI is an open-ended instrument in which the total MRCI score is calculated by summing the scores from each section and where higher total MRCI scores represent more complex medication regimens [31,41,42].
4.5. Statistical Analysis
The data were summarized by location measures (mean, median, minimum and maximum) and dispersion measures (standard error and Interquartile Range, IQR). The variables under study presented a non-gaussian distribution. Quantitative variables were analyzed through the Wilcoxon–Mann–Whitney Test; qualitative variables were analyzed with Pearson’s chi-square test; and the association between two quantitative variables was evaluated with Spearman’s correlation test (and described by the corresponding correlation coefficient). Logistic, linear, simple and multiple regression analysis were conducted, with and without automatic variable selection. Automatic selection was carried out using the Akaike Information Criterion (AIC). The association effect sizes were measured as the odds ratio (OR) or linear coefficient regression (beta). All statistical procedures and analysis were performed with R version 4.3.2. Statistical hypothesis tests with p-values less than 0.05 were considered significant. Confidence intervals are reported with a 95% confidence level.
5. Conclusions
This study demonstrated the existence of high therapeutic complexity in older patients with cancer, suggesting the need for intervention to prevent medication-related problems in this population. The complexity of drug treatment is known to be a risk factor for administration errors and therapeutical non-adherence, which can compromise the safety and effectiveness of the treatment and promote higher healthcare costs, hospital admissions and increased mortality. Older adults with cancer need a regular review and optimization of their prescriptions. The therapeutic review represents an opportunity to deprescribe and simplify medication regimens. Research is needed to better understand the impact of using multiple medications and the effect of medication optimization interventions on clinical outcomes in these patients.
Acknowledgments
V.A. is partially supported by Portuguese funds through CIDMA, The Center for Research and Development in Mathematics and Applications of University of Aveiro, and the Portuguese Foundation for Science and Technology (FCT—Fundação para a Ciência e a Tecnologia), within project UIDB/04106/2020 (https://doi.org/10.54499/UIDB/04106/2020 (accessed on 9 January 2024)).
Author Contributions
Conceptualization, R.F.O. and F.P.; methodology, R.F.O. and O.R.; validation, R.F.O., O.R. and F.P.; formal analysis, R.F.O. and V.A.; investigation, R.F.O.; resources, R.F.O., A.C. and A.I.O.; data curation, R.F.O. and V.A.; writing—original draft preparation, R.F.O., A.I.O. and V.A.; writing—review and editing, R.F.O., A.C., O.R. and F.P.; supervision, O.R., V.A. and F.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of each of the following hospital institutions: Centro Hospitalar do Porto (CES: REF.ª 2014.138(094-DEFI/124-CES)), Centro Hospitalar de São João (CES: 128-14) and Instituto Português de Oncologia do Porto (CES: 183reav/2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data are contained within this article.
Conflicts of Interest
Author Francisco Pimentel was employed by the company BlueClinical. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization Global Health and Aging. Bethesda, MD: National Institute on Aging, National Institutes of Health. World Health Organization. [(accessed on 9 January 2024)];2011 Available online: https://www.nia.nih.gov/sites/default/files/2017-06/global_health_aging.pdf.
- 2.Marengoni A., Angleman S., Melis R., Mangialasche F., Karp A., Garmen A., Meinow B., Fratiglioni L. Aging with multimorbidity: A systematic review of the literature. Ageing Res. Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Khezrian M., McNeil C.J., Murray A.D., Myint P.K. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther. Adv. Drug Saf. 2020;11:2042098620933741. doi: 10.1177/2042098620933741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advinha A.M., de Oliveira-Martins S., Mateus V., Pajote S.G., Lopes M.J. Medication regimen complexity in institutionalized elderly people in an aging society. Int. J. Clin. Pharm. 2014;36:750–756. doi: 10.1007/s11096-014-9963-4. [DOI] [PubMed] [Google Scholar]
- 5.Spinewine A., Schmader K.E., Barber N., Hughes C., Lapane K.L., Swine C., Hanlon J.T. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet. 2007;370:173–184. doi: 10.1016/S0140-6736(07)61091-5. [DOI] [PubMed] [Google Scholar]
- 6.Dixe M.d.A., Pinho J., Pereira F., Verloo H., Meyer-Massetti C., Pereira S.G. Patterns of Medication Management and Associated Medical and Clinical Features Among Home-Dwelling Older Adults: A Cross-Sectional Study in Central Portugal. Int. J. Environ. Res. Public Health. 2023;20:1701. doi: 10.3390/ijerph20031701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajjar E.R., Cafiero A.C., Hanlon J.T. Polypharmacy in elderly patients. Am. J. Geriatr. Pharmacother. 2007;5:345–351. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Advinha A.M., Lopes M.J., de Oliveira-Martins S. Assessment of the elderly’s functional ability to manage their medication: A systematic literature review. Int. J. Clin. Pharm. 2017;39:1–15. doi: 10.1007/s11096-016-0409-z. [DOI] [PubMed] [Google Scholar]
- 9.Wimmer B.C., Johnell K., Fastbom J., Wiese M.D., Bell J.S. Factors associated with medication regimen complexity in older people: A cross-sectional population-based study. Eur. J. Clin. Pharmacol. 2015;71:1099–1108. doi: 10.1007/s00228-015-1883-2. [DOI] [PubMed] [Google Scholar]
- 10.Berben L., Floris G., Wildiers H., Hatse S. Cancer and Aging: Two Tightly Interconnected Biological Processes. Cancers. 2021;13:1400. doi: 10.3390/cancers13061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 12.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 13.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 14.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 15.Lu-Yao G., Nightingale G., Nikita N., Keith S., Gandhi K., Swartz K., Zinner R., Sharma S., Kelly W.M.K., Chapman A. Relationship between polypharmacy and inpatient hospitalization among older adults with cancer treated with intravenous chemotherapy. J. Geriatr. Oncol. 2020;11:579–585. doi: 10.1016/j.jgo.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad N., Lau E.C.Y., Wojt I., Penm J., Dai Z.L., Tan E.C.K. Prevalence of and Risk Factors for Drug-Related Readmissions in Older Adults: A Systematic Review and Meta-Analysis. Drugs Aging. 2024;41:1–11. doi: 10.1007/s40266-023-01076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alves-Conceicao V., Silva D.T.D., Santana V.L., Santos E.G.D., Santos L.M.C., Lyra D.P., Jr. Evaluation of pharmacotherapy complexity in residents of long-term care facilities: A cross-sectional descriptive study. BMC Pharmacol. Toxicol. 2017;18:59. doi: 10.1186/s40360-017-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalic S., Jamsen K.M., Wimmer B.C., Tan E.C., Hilmer S.N., Robson L., Emery T., Bell J.S. Polypharmacy and medication regimen complexity as factors associated with staff informant rated quality of life in residents of aged care facilities: A cross-sectional study. Eur. J. Clin. Pharmacol. 2016;72:1117–1124. doi: 10.1007/s00228-016-2075-4. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira J.M., Galato D., Melo A.C. Medication regimen complexity in adults and the elderly in a primary healthcare setting: Determination of high and low complexities. Pharm. Pract. 2015;13:659. doi: 10.18549/PharmPract.2015.04.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayele A.A., Tegegn H.G., Ayele T.A., Ayalew M.B. Medication regimen complexity and its impact on medication adherence and glycemic control among patients with type 2 diabetes mellitus in an Ethiopian general hospital. BMJ Open Diabetes Res. Care. 2019;7:e000685. doi: 10.1136/bmjdrc-2019-000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhry N.K., Fischer M.A., Avorn J., Liberman J.N., Schneeweiss S., Pakes J., Brennan T.A., Shrank W.H. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch. Intern. Med. 2011;171:814–822. doi: 10.1001/archinternmed.2010.495. [DOI] [PubMed] [Google Scholar]
- 22.Alves-Conceicao V., Rocha K.S.S., Silva F.V.N., Silva R.O.S., Cerqueira-Santos S., Nunes M.A.P., Martins-Filho P.R.S., da Silva D.T., de Lyra D.P., Jr. Are Clinical Outcomes Associated with Medication Regimen Complexity? A Systematic Review and Meta-Analysis. Ann. Pharmacother. 2019;54:301–313. doi: 10.1177/1060028019886846. [DOI] [PubMed] [Google Scholar]
- 23.Alves-Conceicao V., Rocha K.S.S., Silva F.V.N., Silva R.O.S., Silva D.T.D., Lyra D.P., Jr. Medication Regimen Complexity Measured by MRCI: A Systematic Review to Identify Health Outcomes. Ann. Pharmacother. 2018;52:1117–1134. doi: 10.1177/1060028018773691. [DOI] [PubMed] [Google Scholar]
- 24.Wimmer B.C., Cross A.J., Jokanovic N., Wiese M.D., George J., Johnell K., Diug B., Bell J.S. Clinical Outcomes Associated with Medication Regimen Complexity in Older People: A Systematic Review. J. Am. Geriatr. Soc. 2017;65:747–753. doi: 10.1111/jgs.14682. [DOI] [PubMed] [Google Scholar]
- 25.Pantuzza L.L., Ceccato M., Silveira M.R., Junqueira L.M.R., Reis A.M.M. Association between medication regimen complexity and pharmacotherapy adherence: A systematic review. Eur. J. Clin. Pharmacol. 2017;73:1475–1489. doi: 10.1007/s00228-017-2315-2. [DOI] [PubMed] [Google Scholar]
- 26.de Vries S.T., Keers J.C., Visser R., de Zeeuw D., Haaijer-Ruskamp F.M., Voorham J., Denig P. Medication beliefs, treatment complexity, and non-adherence to different drug classes in patients with type 2 diabetes. J. Psychosom. Res. 2014;76:134–138. doi: 10.1016/j.jpsychores.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Tam S.H.Y., Hirsch J.D., Watanabe J.H. Medication Regimen Complexity in Long-Term Care Facilities and Adverse Drug Events-Related Hospitalizations. Consult. Pharm. 2017;32:281–284. doi: 10.4140/TCP.n.2017.281. [DOI] [PubMed] [Google Scholar]
- 28.Frohlich S.E., Zaccolo A.V., da Silva S.L., Mengue S.S. Association between drug prescribing and quality of life in primary care. Pharm. World Sci. PWS. 2010;32:744–751. doi: 10.1007/s11096-010-9431-8. [DOI] [PubMed] [Google Scholar]
- 29.Schoonover H., Corbett C.F., Weeks D.L., Willson M.N., Setter S.M. Predicting potential postdischarge adverse drug events and 30-day unplanned hospital readmissions from medication regimen complexity. J. Patient Saf. 2014;10:186–191. doi: 10.1097/PTS.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 30.Wimmer B.C., Dent E., Visvanathan R., Wiese M.D., Johnell K., Chapman I., Bell J.S. Polypharmacy and medication regimen complexity as factors associated with hospital discharge destination among older people: A prospective cohort study. Drugs Aging. 2014;31:623–630. doi: 10.1007/s40266-014-0185-1. [DOI] [PubMed] [Google Scholar]
- 31.George J., Phun Y.T., Bailey M.J., Kong D.C., Stewart K. Development and validation of the medication regimen complexity index. Ann. Pharmacother. 2004;38:1369–1376. doi: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- 32.Melchiors A.C., Correr C.J., Fernandez-Llimos F. Translation and validation into Portuguese language of the medication regimen complexity index. Arq. Bras. Cardiol. 2007;89:210–218. doi: 10.1590/S0066-782X2007001600001. [DOI] [PubMed] [Google Scholar]
- 33.Stange D., Kriston L., Langebrake C., Cameron L.K., Wollacott J.D., Baehr M., Dartsch D.C. Development and psychometric evaluation of the German version of the Medication Regimen Complexity Index (MRCI-D) J. Eval. Clin. Pract. 2012;18:515–522. doi: 10.1111/j.1365-2753.2011.01636.x. [DOI] [PubMed] [Google Scholar]
- 34.Saez de la Fuente J., Such Diaz A., Canamares-Orbis I., Ramila E., Izquierdo-Garcia E., Esteban C., Escobar-Rodriguez I. Cross-cultural Adaptation and Validation of the Medication Regimen Complexity Index Adapted to Spanish. Ann. Pharmacother. 2016;50:918–925. doi: 10.1177/1060028016656385. [DOI] [PubMed] [Google Scholar]
- 35.Lee S., Jang J., Yang S., Hahn J., Min K.L., Jung E.H., Oh K.S., Cho R., Chang M.J. Development and validation of the Korean version of the medication regimen complexity index. PLoS ONE. 2019;14:e0216805. doi: 10.1371/journal.pone.0216805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuyan B., Babi B., Sancar M., Ay P., Yucel E., Yucel A., Izzettin F.V. Validation of the Turkish version of medication regimen complexity index among elderly patients. J. Eval. Clin. Pract. 2016;22:732–736. doi: 10.1111/jep.12526. [DOI] [PubMed] [Google Scholar]
- 37.Falch C., Alves G. Pharmacists’ Role in Older Adults’ Medication Regimen Complexity: A Systematic Review. Int. J. Environ. Res. Public Health. 2021;18:8824. doi: 10.3390/ijerph18168824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brysch E.G., Cauthon K.A.B., Kalich B.A., Sarbacker G.B. Medication Regimen Complexity Index in the Elderly in an Outpatient Setting: A Literature Review. Consult. Pharm. J. Am. Soc. Consult. Pharm. 2018;33:484–496. doi: 10.4140/TCP.n.2018.484. [DOI] [PubMed] [Google Scholar]
- 39.Paquin A.M., Zimmerman K.M., Kostas T.R., Pelletier L., Hwang A., Simone M., Skarf L.M., Rudolph J.L. Complexity perplexity: A systematic review to describe the measurement of medication regimen complexity. Expert. Opin. Drug Saf. 2013;12:829–840. doi: 10.1517/14740338.2013.823944. [DOI] [PubMed] [Google Scholar]
- 40.Pantuzza L.L., Ceccato M., Silveira M.R., Pinto I.V., Reis A.M.M. Validation and standardization of the Brazilian version of the Medication Regimen Complexity Index for older adults in primary care. Geriatr. Gerontol. Int. 2018;18:853–859. doi: 10.1111/ggi.13261. [DOI] [PubMed] [Google Scholar]
- 41.Mansur N., Weiss A., Beloosesky Y. Looking beyond polypharmacy: Quantification of medication regimen complexity in the elderly. Am. J. Geriatr. Pharmacother. 2012;10:223–229. doi: 10.1016/j.amjopharm.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Hirsch J.D., Metz K.R., Hosokawa P.W., Libby A.M. Validation of a patient-level medication regimen complexity index as a possible tool to identify patients for medication therapy management intervention. Pharmacotherapy. 2014;34:826–835. doi: 10.1002/phar.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Libby A.M., Fish D.N., Hosokawa P.W., Linnebur S.A., Metz K.R., Nair K.V., Saseen J.J., Vande Griend J.P., Vu S.P., Hirsch J.D. Patient-level medication regimen complexity across populations with chronic disease. Clin. Ther. 2013;35:385–398.e1. doi: 10.1016/j.clinthera.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Herledan C., Toulemonde A., Clairet A.L., Boulin M., Falandry C., De Decker L., Rioufol C., Bayle A., Bertrand N. Enhancing collaboration between geriatricians, oncologists, and pharmacists to optimize medication therapy in older adults with cancer: A position paper from SOFOG-SFPO. Crit. Rev. Oncol. Hematol. 2023;190:104117. doi: 10.1016/j.critrevonc.2023.104117. [DOI] [PubMed] [Google Scholar]
- 45.Wimmer B.C., Bell J.S., Fastbom J., Wiese M.D., Johnell K. Medication Regimen Complexity and Polypharmacy as Factors Associated with All-Cause Mortality in Older People: A Population-Based Cohort Study. Ann. Pharmacother. 2016;50:89–95. doi: 10.1177/1060028015621071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen E.Y.H., Zhao J.X., Ilomäki J., Sluggett J.K., Bell J.S., Wimmer B.C., Hilmer S.N., Blais J.E., Wong I.C.K., Chan E.W. Medication Regimen Complexity and Risk of Bleeding in People Who Initiate Oral Anticoagulants for Atrial Fibrillation: A Population-Based Study. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2023;78:470–478. doi: 10.1093/gerona/glac203. [DOI] [PubMed] [Google Scholar]
- 47.Krska J., Corlett S.A., Katusiime B. Complexity of Medicine Regimens and Patient Perception of Medicine Burden. Pharmacy. 2019;7:18. doi: 10.3390/pharmacy7010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cobretti M.R., Page R.L., 2nd, Linnebur S.A., Deininger K.M., Ambardekar A.V., Lindenfeld J., Aquilante C.L. Medication regimen complexity in ambulatory older adults with heart failure. Clin. Interv. Aging. 2017;12:679–686. doi: 10.2147/CIA.S130832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tesfaye W.H., Peterson G.M., Castelino R.L., McKercher C., Jose M., Zaidi S.T.R., Wimmer B.C. Medication-Related Factors and Hospital Readmission in Older Adults with Chronic Kidney Disease. J. Clin. Med. 2019;8:395. doi: 10.3390/jcm8030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wimmer B.C., Bell J.S., Fastbom J., Wiese M.D., Johnell K. Medication Regimen Complexity and Number of Medications as Factors Associated with Unplanned Hospitalizations in Older People: A Population-Based Cohort Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016;71:831–837. doi: 10.1093/gerona/glv219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abada S., Clark L.E., Sinha A.K., Xia R., Pace-Murphy K., Flores R.J., Burnett J. Medication Regimen Complexity and Low Adherence in Older Community-Dwelling Adults with Substantiated Self-Neglect. J. Appl. Gerontol. Off. J. South. Gerontol. Soc. 2019;38:866–883. doi: 10.1177/0733464817714565. [DOI] [PubMed] [Google Scholar]
- 52.Chang W.T., Kowalski S.R., Sorich W., Alderman C.P. Medication regimen complexity and prevalence of potentially inappropriate medicines in older patients after hospitalisation. Int. J. Clin. Pharm. 2017;39:867–873. doi: 10.1007/s11096-017-0490-y. [DOI] [PubMed] [Google Scholar]
- 53.Linnebur S.A., Vande Griend J.P., Metz K.R., Hosokawa P.W., Hirsch J.D., Libby A.M. Patient-level medication regimen complexity in older adults with depression. Clin. Ther. 2014;36:1538–1546.e1. doi: 10.1016/j.clinthera.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Bellostas-Munoz L., Diez-Manglano J. Complexity of the medication regimen for polypathological patients. Rev. Clin. Esp. 2018;218:342–350. doi: 10.1016/j.rceng.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Spargo M., Ryan C., Downey D., Hughes C. The association between polypharmacy and medication regimen complexity and antibiotic use in bronchiectasis. Int. J. Clin. Pharm. 2018;40:1342–1348. doi: 10.1007/s11096-018-0681-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newsome A.S., Smith S.E., Olney W.J., Jones T.W., Forehand C.C., Jun A.H., Coppiano L. Medication regimen complexity is associated with pharmacist interventions and drug-drug interactions: A use of the novel MRC-ICU scoring tool. J. Am. Coll. Clin. Pharm. 2020;3:47–56. doi: 10.1002/jac5.1146. [DOI] [Google Scholar]
- 57.Rettig S.M., Wood Y., Hirsch J.D. Medication regimen complexity in patients with uncontrolled hypertension and/or diabetes. J. Am. Pharm. Assoc. 2013;53:427–431. doi: 10.1331/JAPhA.2013.13003. [DOI] [PubMed] [Google Scholar]
- 58.Ab Rahman N., Lim M.T., Thevendran S., Ahmad Hamdi N., Sivasampu S. Medication Regimen Complexity and Medication Burden Among Patients with Type 2 Diabetes Mellitus: A Retrospective Analysis. Front. Pharmacol. 2022;13:808190. doi: 10.3389/fphar.2022.808190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Budnitz D.S., Lovegrove M.C., Shehab N., Richards C.L. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 60.Gnjidic D., Hilmer S.N., Hartikainen S., Tolppanen A.M., Taipale H., Koponen M., Bell J.S. Impact of high risk drug use on hospitalization and mortality in older people with and without Alzheimer’s disease: A national population cohort study. PLoS ONE. 2014;9:e83224. doi: 10.1371/journal.pone.0083224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tesfaye W.H., Peterson G.M., Castelino R.L., McKercher C., Jose M.D., Wimmer B.C., Zaidi S.T.R. Medication Regimen Complexity and Hospital Readmission in Older Adults with Chronic Kidney Disease. Ann. Pharmacother. 2019;53:28–34. doi: 10.1177/1060028018793419. [DOI] [PubMed] [Google Scholar]
- 62.Pantuzza L.L.N., das Graças Braga Ceccato M., Reis E.A., Silveira M.R., Almeida-Brasil C.C., Almeida T.A., Pinto I.V.L., Reis A.M.M. Factors associated with high medication regimen complexity in primary care older adults in Brazil. Eur. Geriatr. Med. 2020;11:279–287. doi: 10.1007/s41999-019-00275-0. [DOI] [PubMed] [Google Scholar]
- 63.Bryant B.M., Libby A.M., Metz K.R., Page R.L., 2nd, Ambardekar A.V., Lindenfeld J., Aquilante C.L. Evaluating Patient-Level Medication Regimen Complexity over Time in Heart Transplant Recipients. Ann. Pharmacother. 2016;50:926–934. doi: 10.1177/1060028016657552. [DOI] [PubMed] [Google Scholar]
- 64.Metz K.R., Fish D.N., Hosokawa P.W., Hirsch J.D., Libby A.M. Patient-Level Medication Regimen Complexity in Patients with HIV. Ann. Pharmacother. 2014;48:1129–1137. doi: 10.1177/1060028014539642. [DOI] [PubMed] [Google Scholar]
- 65.Colavecchia A.C., Putney D.R., Johnson M.L., Aparasu R.R. Discharge medication complexity and 30-day heart failure readmissions. Res. Soc. Adm. Pharm. 2017;13:857–863. doi: 10.1016/j.sapharm.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Olson C.H., Dey S., Kumar V., Monsen K.A., Westra B.L. Clustering of elderly patient subgroups to identify medication-related readmission risks. Int. J. Med. Inform. 2016;85:43–52. doi: 10.1016/j.ijmedinf.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Lalic S., Sluggett J.K., Ilomaki J., Wimmer B.C., Tan E.C., Robson L., Emery T., Bell J.S. Polypharmacy and Medication Regimen Complexity as Risk Factors for Hospitalization Among Residents of Long-Term Care Facilities: A Prospective Cohort Study. J. Am. Med. Dir. Assoc. 2016;17:1067.e1–1067.e6. doi: 10.1016/j.jamda.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 68.Willson M.N., Greer C.L., Weeks D.L. Medication regimen complexity and hospital readmission for an adverse drug event. Ann. Pharmacother. 2014;48:26–32. doi: 10.1177/1060028013510898. [DOI] [PubMed] [Google Scholar]
- 69.Stange D., Kriston L., von-Wolff A., Baehr M., Dartsch D.C. Reducing cardiovascular medication complexity in a German university hospital: Effects of a structured pharmaceutical management intervention on adherence. J. Manag. Care Pharm. JMCP. 2013;19:396–407. doi: 10.18553/jmcp.2013.19.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McDonald M.V., Peng T.R., Sridharan S., Foust J.B., Kogan P., Pezzin L.E., Feldman P.H. Automating the medication regimen complexity index. J. Am. Med. Inform. Assoc. 2013;20:499–505. doi: 10.1136/amiajnl-2012-001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clay P.G. Medication regimen complexity indices: A tool to focus MTM efforts? J. Am. Pharm. Assoc. 2014;54:664. doi: 10.1331/JAPhA.2014.14542. [DOI] [PubMed] [Google Scholar]
- 72.Apóstolo J.L.A., Paiva D.D.S., Silva R., Santos E., Schultz T.J. Adaptation and validation into Portuguese language of the six-item cognitive impairment test (6CIT) Aging Ment. Health. 2018;22:1184–1189. doi: 10.1080/13607863.2017.1348473. [DOI] [PubMed] [Google Scholar]
- 73.Hong S., Lee J.H., Chun E.K., Kim K.I., Kim J.W., Kim S.H., Lee Y.G., Hwang I.G., Kim J.Y., Koh S.J., et al. Polypharmacy, Inappropriate Medication Use, and Drug Interactions in Older Korean Patients with Cancer Receiving First-Line Palliative Chemotherapy. Oncologist. 2020;25:e502–e511. doi: 10.1634/theoncologist.2019-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maggiore R.J., Dale W., Gross C.P., Feng T., Tew W.P., Mohile S.G., Owusu C., Klepin H.D., Lichtman S.M., Gajra A., et al. Polypharmacy and potentially inappropriate medication use in older adults with cancer undergoing chemotherapy: Effect on chemotherapy-related toxicity and hospitalization during treatment. J. Am. Geriatr. Soc. 2014;62:1505–1512. doi: 10.1111/jgs.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prithviraj G.K., Koroukian S., Margevicius S., Berger N.A., Bagai R., Owusu C. Patient Characteristics Associated with Polypharmacy and Inappropriate Prescribing of Medications Among Older Adults with Cancer. J. Geriatr. Oncol. 2012;3:228–237. doi: 10.1016/j.jgo.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maggiore R.J., Gross C.P., Hurria A. Polypharmacy in older adults with cancer. Oncologist. 2010;15:507–522. doi: 10.1634/theoncologist.2009-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schöttker B., Saum K.U., Muhlack D.C., Hoppe L.K., Holleczek B., Brenner H. Polypharmacy and mortality: New insights from a large cohort of older adults by detection of effect modification by multi-morbidity and comprehensive correction of confounding by indication. Eur. J. Clin. Pharmacol. 2017;73:1041–1048. doi: 10.1007/s00228-017-2266-7. [DOI] [PubMed] [Google Scholar]
- 78.Micromedex® Drug Interaction Checking (Electronic Version) Health. [(accessed on 9 January 2016)]. Available online: https://www.micromedexsolutions.com/home/dispatch/
- 79.Bossaer J.B., Thomas C.M. Drug Interaction Database Sensitivity with Oral Antineoplastics: An Exploratory Analysis. J. Oncol. Pract. 2017;13:e217–e222. doi: 10.1200/JOP.2016.016212. [DOI] [PubMed] [Google Scholar]
- 80.Yap K.Y.-L., Raaj S., Chan A. OncoRx-IQ: A tool for quality assessment of online anticancer drug interactions. Int. J. Qual. Health Care. 2010;22:93–106. doi: 10.1093/intqhc/mzq004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within this article.