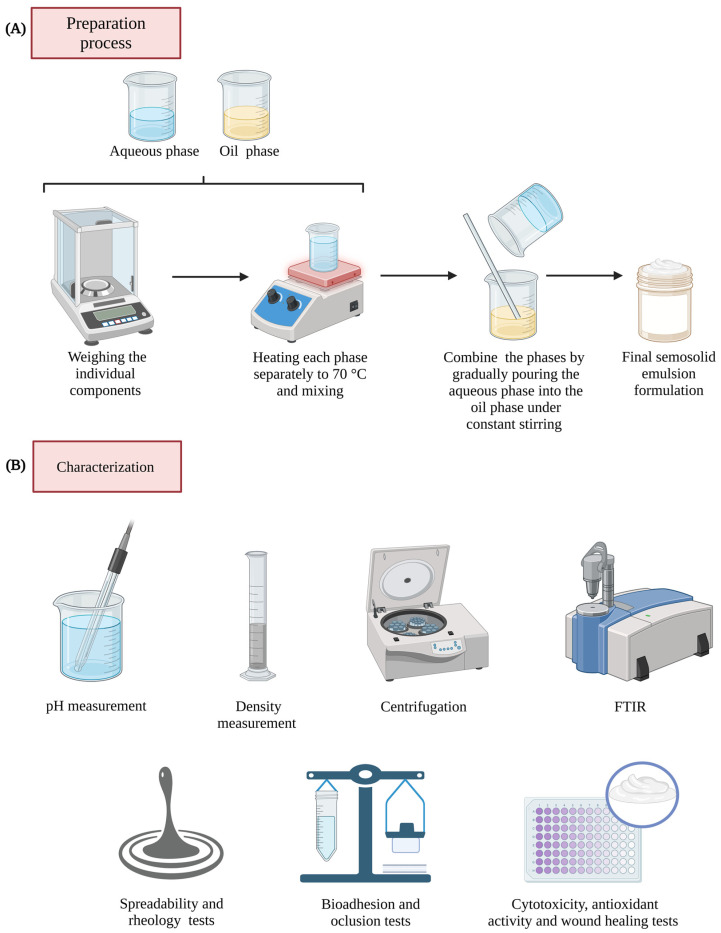
Figure 1.
Flowchart of the formulation and characterization procedures. The preparation of the semisolid emulsion involves several sequential steps (A): weighing the individual components for the oil phase (OP) and aqueous phase (AP), heating each phase separately to 70 °C to ensure proper dissolution and mixing, combining the phases by gradually pouring the aqueous phase (AP) into the oil phase (OP) under constant stirring to form a uniform emulsion, and obtaining the final gel–cream formulation. The emulsion was subsequently characterized through various analyses (B): Fourier-transform infrared spectroscopy (FTIR) to assess molecular interactions and confirm compatibility among components, centrifugation to evaluate physical stability and detect any phase separation, spreadability and reology testing to determine ease of application and coverage on the skin, density measurement to assess formulation consistency, pH measurement with a pH meter to ensure suitability for skin application, bioadhesion and occlusion potential, antioxidant activity, cytotoxicity testing using cell cultures to evaluate biocompatibility and potential safety for skin use, and wound healing assay to determine efficacy.