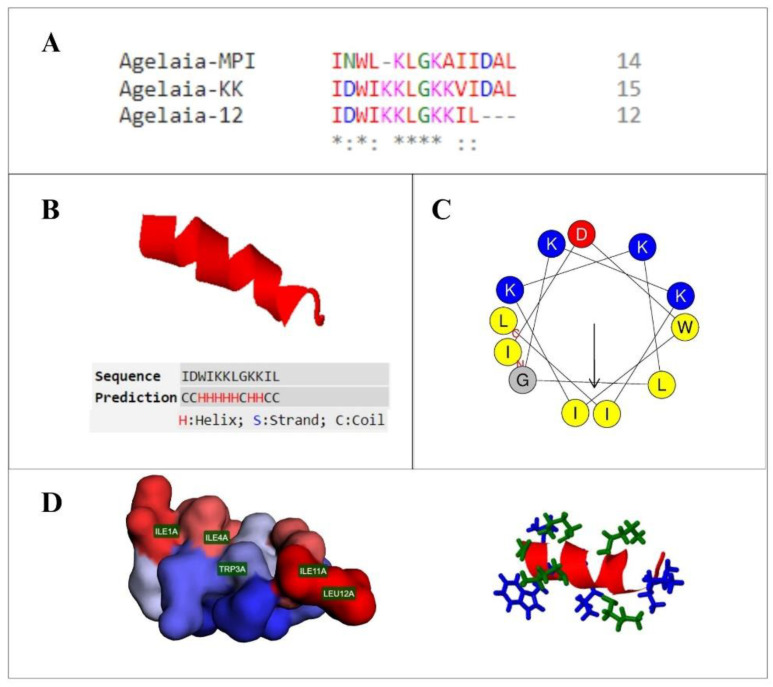
Figure 1.
Structure of the antimicrobial peptide Agelaia-12. (A) Multiple alignment of the natural peptide Agelaia-MPI and derived synthetic peptides, Agelaia-KK and Agelaia-12. (*) identical amino acids, conserved region and (:) semiconservative amino acids substitutions. (B) Three-dimensional secondary structure of the peptide Agelaia-12, demonstrating its predominant alpha helix secondary structure. (C) Diagram of the helical wheel of the Agelaia-12 peptide, with a region composed of polar and cationic residues, in blue, and nonpolar residues, which are mostly hydrophobic, in yellow. The arrow indicates the direction of the hydrophobic moment of the molecule. (D) On the left, a representation of the polar (in blue) and nonpolar (in red) surfaces of the peptide is demonstrated, highlighting the location of nonpolar residues, and on the right side, a representation of the side chains of charged (green) and uncharged (blue) residues.