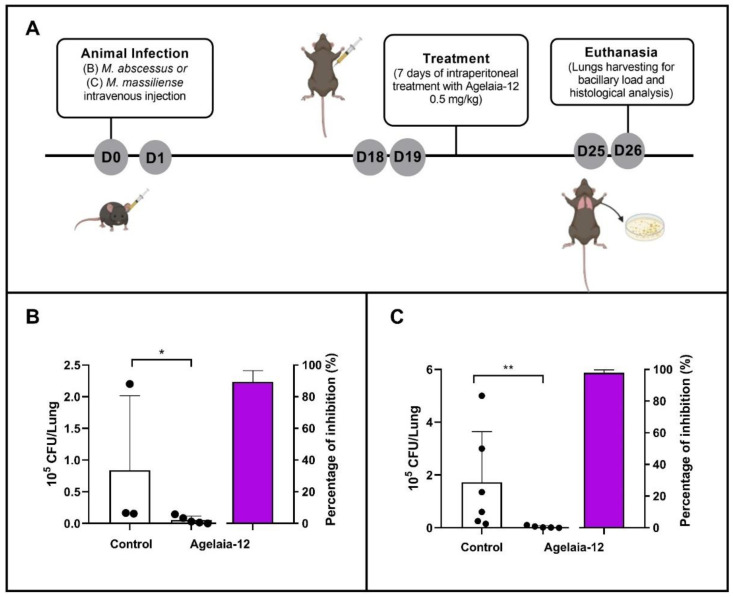
Figure 6.
In vivo assay for evaluation of Agelaia-12 treatment of IFN-y KO infected mice. (A) Timeline of the in vivo assay conducted with female and male IFN-y KO mice (day zero: infection of the animals with 106 CFU/mL of M. abscessus or 108 CFU/mL of M. massiliense intravenously). On day nineteen (D19), animals were treated with PBS (control group) or with Agelaia-12 intraperitoneally for seven days. On day twenty-six (D26), animals were euthanized for lung collection and analysis of the bacterial load and histological studies. (B,C) Mycobacterial load in the lungs of the control group and those treated with Agelaia-12 with M. abscessus ATCC (B) or M. massiliense GO06 (C). The far right column in each graph corresponds to the growth inhibition percentage by Agelaia-12 treatment. * Significant differences (p < 0.05); ** significant differences (p < 0.001).