Abstract
1. In the whole-cell configuration of the patch clamp technique, isolated rat carotid body type I cells exhibited reversible activation of Cl- currents during cell swelling effected by hypotonic extracellular solutions. 2. Hypotonic solutions evoked outwardly rectifying, non-inactivating currents which showed time-independent activation. The reversal potential (E(rev)) for the hypotonically evoked current was 1.6 +/- 0.6 mV (n = 26). Reduction of extracellular Cl- from 133 to 65.5 mM caused a shift in E(rev) of +14.7 +/- 0.4 mV (n = 5). 3. The swelling-activated Cl- current could not activate when ATP was omitted from the patch pipette or when substituted for the non-hydrolysable ATP analogues 5'-adenylylimidodiphosphate, AMP-PNP (2 mM) or beta, gamma-methylene-adenosine 5'-triphosphate. AMP-PCP (2 mM). The current also failed to activate in the absence of free intracellular Ca2+. 4. The swelling-activated Cl- current was sensitive to blockade by the Cl- channel blockers niflumic acid (300 microM) and 4,4'-diisothiocyanatostilbene-2, 2'-disulphonic acid (DIDS; 200 microM), although the blockade by DIDS was voltage dependent. 5. A similar, non-inactivating, outwardly rectifying Cl- current was evoked by the inclusion of cAMP (200 microM) in the patch pipette. This current could be inhibited by niflumic acid (300 microM), DIDS (200 microM) and hypertonic solutions, and was virtually abolished in the absence of intracellular ATP. 6. In conclusion, carotid body type I cells possess Cl- currents activated by cell swelling and rises in intracellular cAMP concentration. These currents may be involved in cell volume regulation, blood volume and osmolarity regulation and the response of the type I cell to chemostimuli.
Full text
PDF

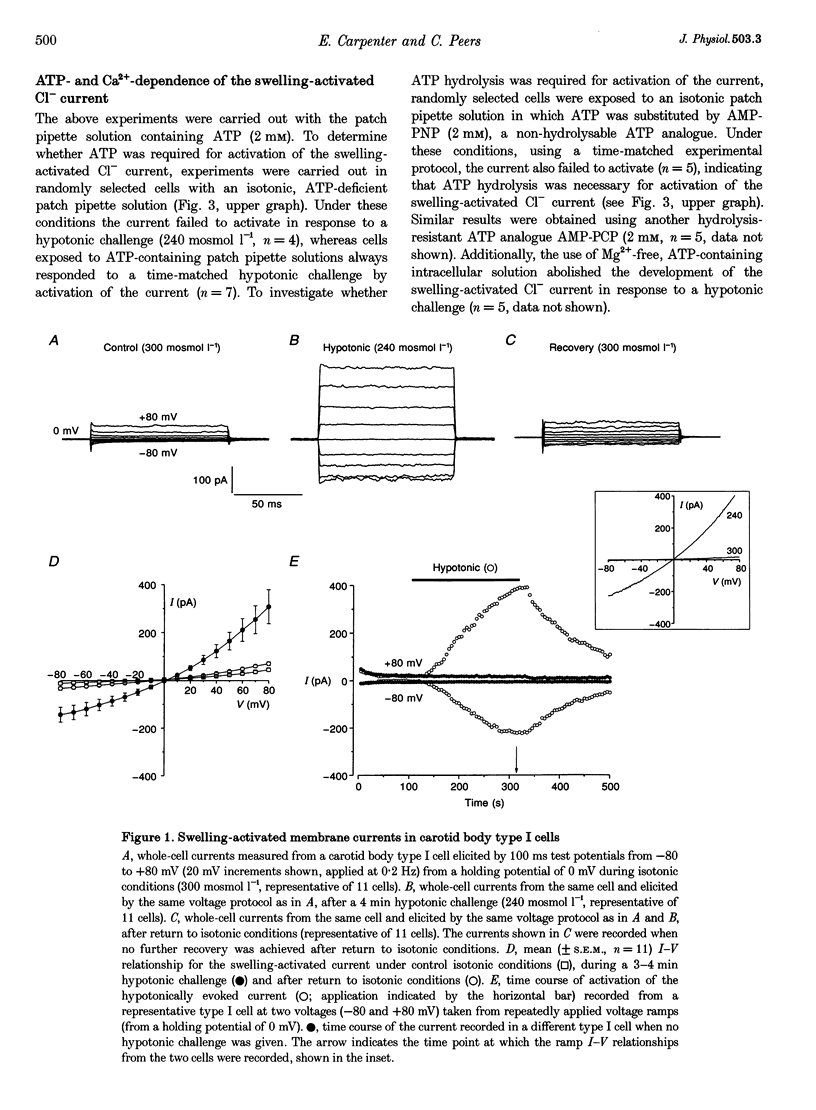
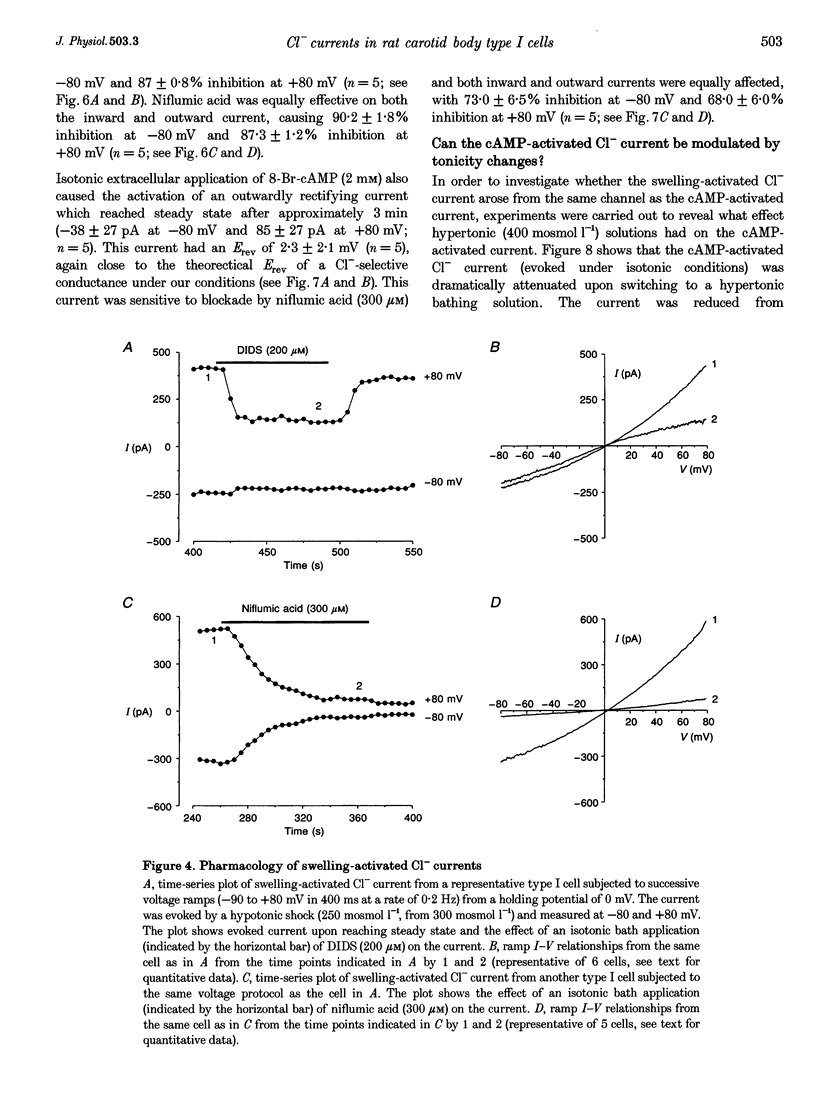
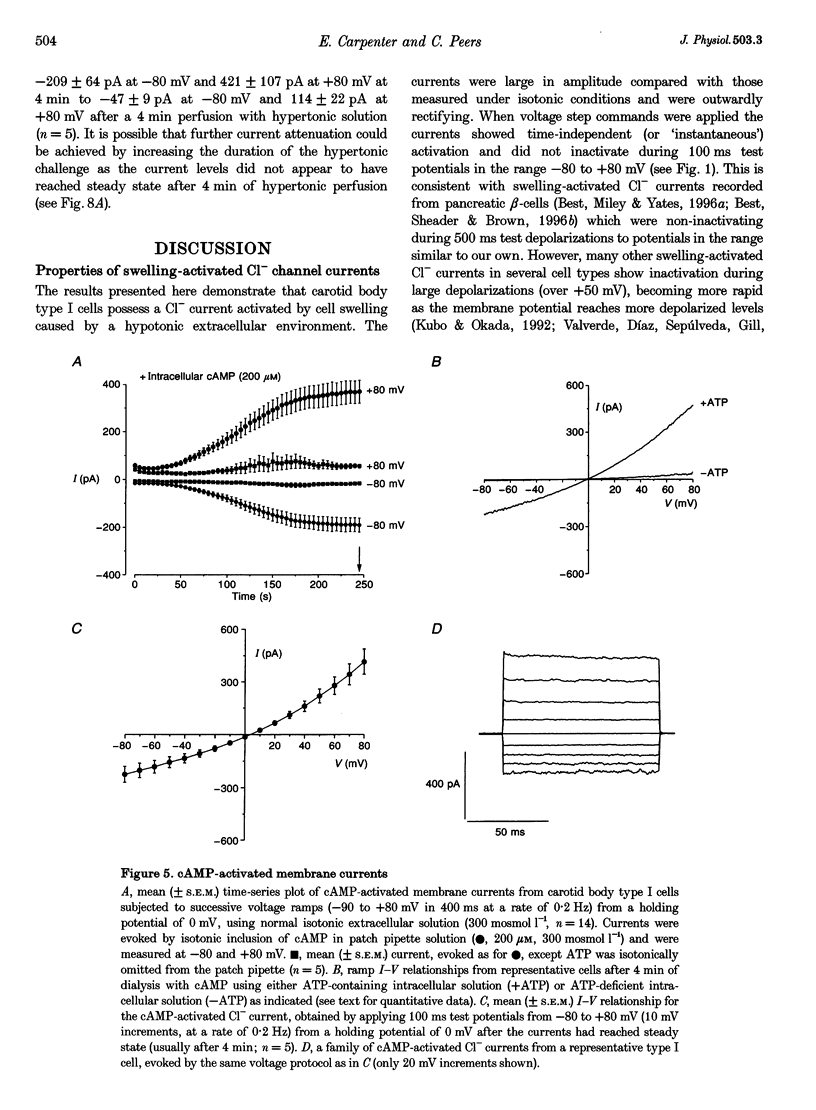
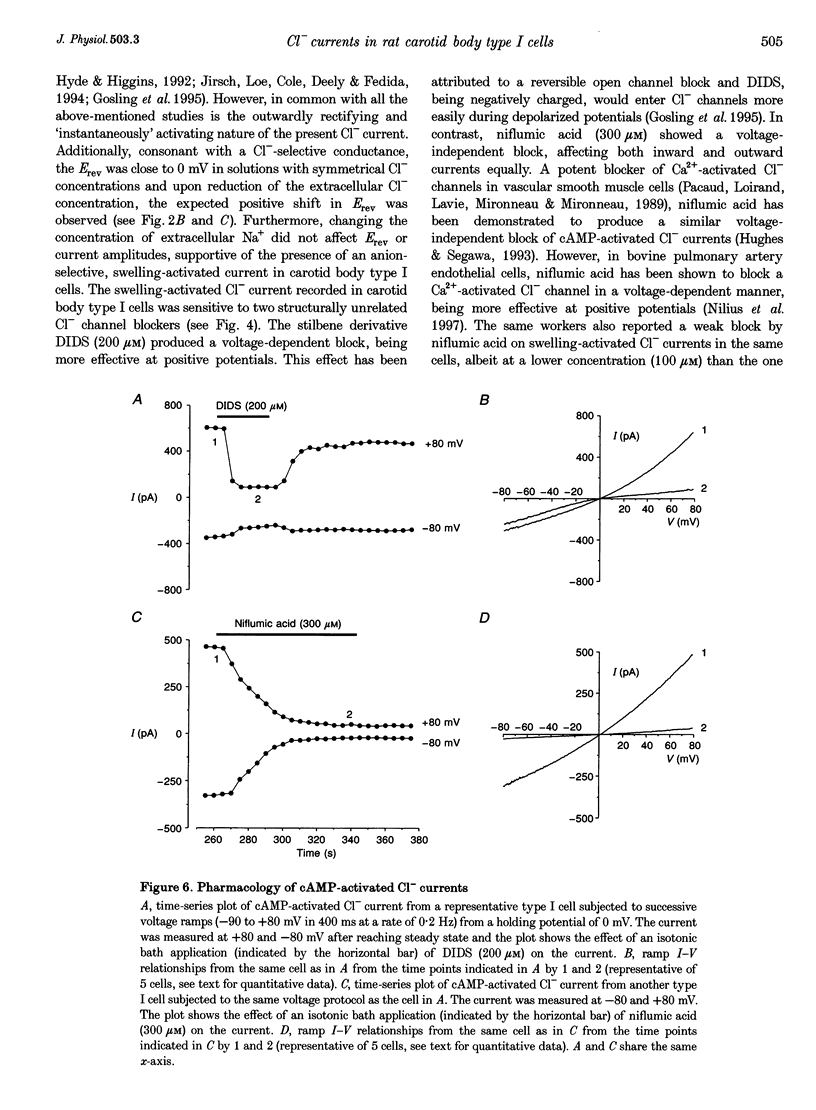
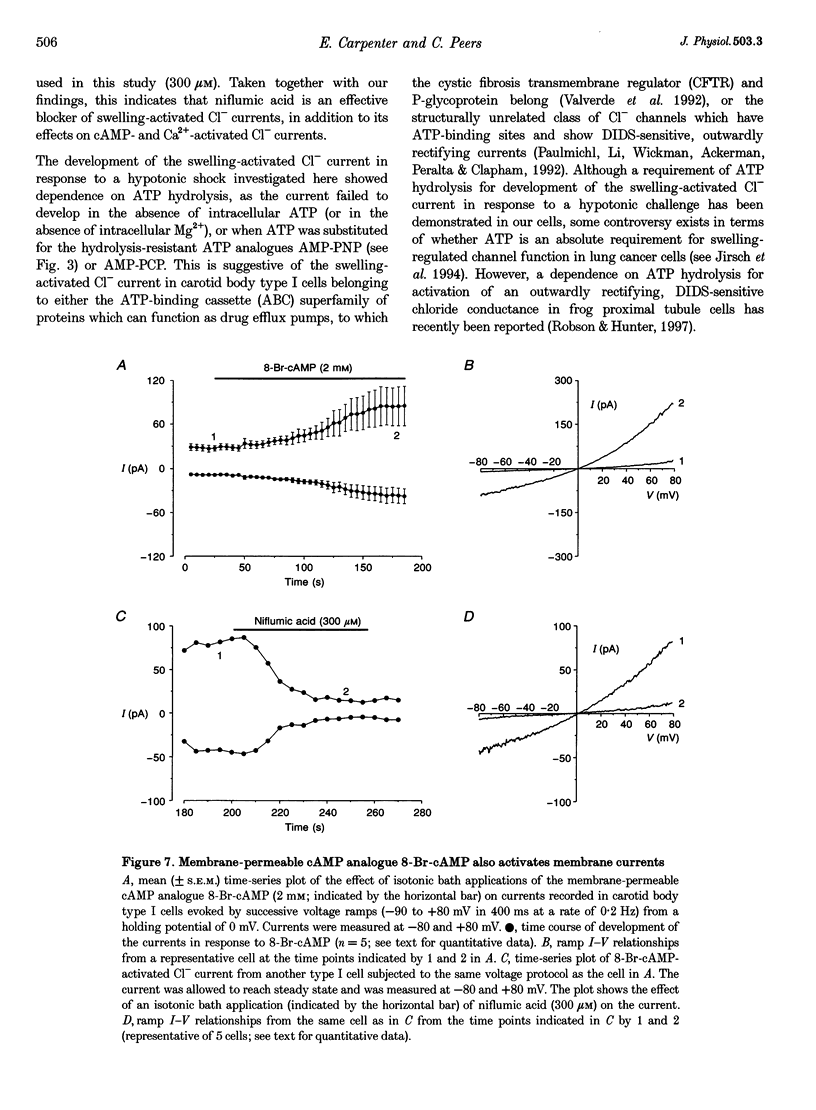




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best L., Miley H. E., Yates A. P. Activation of an anion conductance and beta-cell depolarization during hypotonically induced insulin release. Exp Physiol. 1996 Nov;81(6):927–933. doi: 10.1113/expphysiol.1996.sp003993. [DOI] [PubMed] [Google Scholar]
- Best L., Sheader E. A., Brown P. D. A volume-activated anion conductance in insulin-secreting cells. Pflugers Arch. 1996 Jan;431(3):363–370. doi: 10.1007/BF02207273. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. J Physiol. 1994 Jul 1;478(Pt 1):157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachero T. G., Rigual R., Rocher A., Gonzalez C. Cholera and pertussis toxins reveal multiple regulation of cAMP levels in the rabbit carotid body. Eur J Neurosci. 1996 Nov;8(11):2320–2327. doi: 10.1111/j.1460-9568.1996.tb01195.x. [DOI] [PubMed] [Google Scholar]
- Delpiano M. A., Acker H. Hypoxia increases the cyclic AMP content of the cat carotid body in vitro. J Neurochem. 1991 Jul;57(1):291–297. doi: 10.1111/j.1471-4159.1991.tb02127.x. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R., Eyzaguirre C., Monti-Bloch L. Thermal and osmotic responses of arterial receptors. J Neurophysiol. 1979 May;42(3):665–680. doi: 10.1152/jn.1979.42.3.665. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Almaraz L., Obeso A., Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994 Oct;74(4):829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gosling M., Smith J. W., Poyner D. R. Characterization of a volume-sensitive chloride current in rat osteoblast-like (ROS 17/2.8) cells. J Physiol. 1995 Jun 15;485(Pt 3):671–682. doi: 10.1113/jphysiol.1995.sp020761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hatton C. J., Peers C. Hypoxic inhibition of K+ currents in isolated rat type I carotid body cells: evidence against the involvement of cyclic nucleotides. Pflugers Arch. 1996 Nov-Dec;433(1-2):129–135. doi: 10.1007/s004240050258. [DOI] [PubMed] [Google Scholar]
- He S. F., Wei J. Y., Eyzaguirre C. Influence of intracellular pH on the membrane potential of cultured carotid body glomus cells. Brain Res. 1993 Jan 22;601(1-2):353–357. doi: 10.1016/0006-8993(93)91736-c. [DOI] [PubMed] [Google Scholar]
- Hughes B. A., Segawa Y. cAMP-activated chloride currents in amphibian retinal pigment epithelial cells. J Physiol. 1993 Jul;466:749–766. [PMC free article] [PubMed] [Google Scholar]
- Jirsch J. D., Loe D. W., Cole S. P., Deeley R. G., Fedida D. ATP is not required for anion current activated by cell swelling in multidrug-resistant lung cancer cells. Am J Physiol. 1994 Sep;267(3 Pt 1):C688–C699. doi: 10.1152/ajpcell.1994.267.3.C688. [DOI] [PubMed] [Google Scholar]
- Kubo M., Okada Y. Volume-regulatory Cl- channel currents in cultured human epithelial cells. J Physiol. 1992 Oct;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López J. R., De Luis D. A., Gonzalez C. Properties of a transient K+ current in chemoreceptor cells of rabbit carotid body. J Physiol. 1993 Jan;460:15–32. doi: 10.1113/jphysiol.1993.sp019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Oike M., Zahradnik I., Droogmans G. Activation of a Cl- current by hypotonic volume increase in human endothelial cells. J Gen Physiol. 1994 May;103(5):787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Prenen J., Szücs G., Wei L., Tanzi F., Voets T., Droogmans G. Calcium-activated chloride channels in bovine pulmonary artery endothelial cells. J Physiol. 1997 Jan 15;498(Pt 2):381–396. doi: 10.1113/jphysiol.1997.sp021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama Y., Walker J. L., Eyzaguirre C. The intracellular chloride activity of glomus cells in the isolated rabbit carotid body. Brain Res. 1986 Mar 12;368(1):167–169. doi: 10.1016/0006-8993(86)91056-5. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Lavie J. L., Mironneau C., Mironneau J. Calcium-activated chloride current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1989 Apr;413(6):629–636. doi: 10.1007/BF00581813. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Li Y., Wickman K., Ackerman M., Peralta E., Clapham D. New mammalian chloride channel identified by expression cloning. Nature. 1992 Mar 19;356(6366):238–241. doi: 10.1038/356238a0. [DOI] [PubMed] [Google Scholar]
- Peers C., Buckler K. J. Transduction of chemostimuli by the type I carotid body cell. J Membr Biol. 1995 Mar;144(1):1–9. doi: 10.1007/BF00238411. [DOI] [PubMed] [Google Scholar]
- Peers C., Green F. K. Inhibition of Ca(2+)-activated K+ currents by intracellular acidosis in isolated type I cells of the neonatal rat carotid body. J Physiol. 1991 Jun;437:589–602. doi: 10.1113/jphysiol.1991.sp018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-García M. T., Almaraz L., González C. Cyclic AMP modulates differentially the release of dopamine induced by hypoxia and other stimuli and increases dopamine synthesis in the rabbit carotid body. J Neurochem. 1991 Dec;57(6):1992–2000. doi: 10.1111/j.1471-4159.1991.tb06414.x. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Almaraz L., González C. Effects of different types of stimulation on cyclic AMP content in the rabbit carotid body: functional significance. J Neurochem. 1990 Oct;55(4):1287–1293. doi: 10.1111/j.1471-4159.1990.tb03137.x. [DOI] [PubMed] [Google Scholar]
- Robson L., Hunter M. Regulation of an outwardly rectifying Cl- conductance in single proximal tubule cells isolated from frog kidney. J Physiol. 1997 Jan 15;498(Pt 2):409–417. doi: 10.1113/jphysiol.1997.sp021867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson L., Hunter M. Volume regulatory responses in frog isolated proximal cells. Pflugers Arch. 1994 Aug;428(1):60–68. doi: 10.1007/BF00374752. [DOI] [PubMed] [Google Scholar]
- Schwiebert E. M., Flotte T., Cutting G. R., Guggino W. B. Both CFTR and outwardly rectifying chloride channels contribute to cAMP-stimulated whole cell chloride currents. Am J Physiol. 1994 May;266(5 Pt 1):C1464–C1477. doi: 10.1152/ajpcell.1994.266.5.C1464. [DOI] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Chloride channels in cultured glomus cells of the rat carotid body. Am J Physiol. 1989 Aug;257(2 Pt 1):C174–C181. doi: 10.1152/ajpcell.1989.257.2.C174. [DOI] [PubMed] [Google Scholar]
- Szücs G., Heinke S., Droogmans G., Nilius B. Activation of the volume-sensitive chloride current in vascular endothelial cells requires a permissive intracellular Ca2+ concentration. Pflugers Arch. 1996 Jan;431(3):467–469. doi: 10.1007/BF02207289. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Díaz M., Sepúlveda F. V., Gill D. R., Hyde S. C., Higgins C. F. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992 Feb 27;355(6363):830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- Vandenberg J. I., Yoshida A., Kirk K., Powell T. Swelling-activated and isoprenaline-activated chloride currents in guinea pig cardiac myocytes have distinct electrophysiology and pharmacology. J Gen Physiol. 1994 Dec;104(6):997–1017. doi: 10.1085/jgp.104.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. J., Cheng G. F., Dinger B. G., Fidone S. J. Effects of hypoxia on cyclic nucleotide formation in rabbit carotid body in vitro. Neurosci Lett. 1989 Oct 23;105(1-2):164–168. doi: 10.1016/0304-3940(89)90030-x. [DOI] [PubMed] [Google Scholar]
- Wang W. J., Cheng G. F., Yoshizaki K., Dinger B., Fidone S. The role of cyclic AMP in chemoreception in the rabbit carotid body. Brain Res. 1991 Feb 1;540(1-2):96–104. doi: 10.1016/0006-8993(91)90495-h. [DOI] [PubMed] [Google Scholar]
- Wyatt C. N., Peers C. Ca(2+)-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J Physiol. 1995 Mar 15;483(Pt 3):559–565. doi: 10.1113/jphysiol.1995.sp020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt C. N., Wright C., Bee D., Peers C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):295–299. doi: 10.1073/pnas.92.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H. Effect of baro- and chemoreceptor activation on supraoptic nuclei neurons in the hypothalamus. Brain Res. 1977 May 13;126(3):551–556. doi: 10.1016/0006-8993(77)90607-2. [DOI] [PubMed] [Google Scholar]


