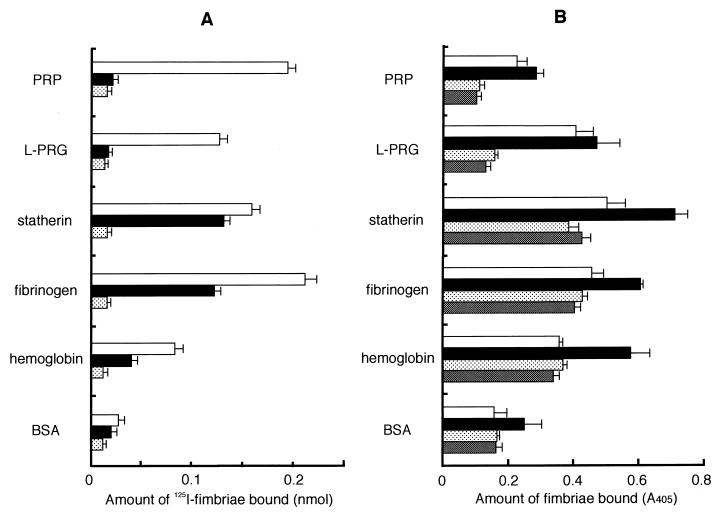
FIG. 3.
Binding of P. gingivalis fimbriae to the host proteins on an apatitic or a polystyrene surface. (A) Binding abilities of fimbriae to host proteins were assayed by using HA beads. 125I-labeled fimbriae (0.5 nmol) was added to a tube containing host protein-coated HA beads and incubated at RT for 1 h. 125I-labeled fimbriae (0.5 nmol) added (□); 125I-labeled fimbriae (0.5 nmol) plus peptide PRP-C (50 nmol) added (■); fimbriae (0.5 nmol) plus nonlabeled fimbriae (25 nmol) added to obtain the nonspecific binding level ( ). The specific binding level was calculated by subtracting the nonspecific binding level. Significant differences (P < 0.01) were observed among fimbrial binding levels (□) to host proteins in the absence of peptide PRP-C. (B) Binding assay performed with polystyrene surface. The wells of 96-well microtiter plates were coated with host proteins or BSA (100 μl of 0.1 mg/ml in PBS) at 37°C for 2 h. An aliquot of fimbriae and, if necessary, peptide PRP-C as an inhibitor was added to the wells and then incubated at RT for 1 h. Rabbit anti-fimbriae immunoglobulin G (1:1,000) was added to detect the amount of bound fimbriae. □, 12.2 pmol of added fimbriae; ■, 24.4 pmol of added fimbriae; , 12.2 pmol of fimbriae plus 100 pmol of added peptide PRP-C; ▩, 12.2 pmol of fimbriae plus 1 nmol of added peptide PRP-C. Significant differences (P < 0.01) were observed among fimbrial binding levels (■) to host proteins in the absence of peptide PRP-C and between PRP and BSA and between fibrinogen and hemoglobin. All assays were performed in triplicate, on three separate occasions.