Abstract
Vascular pathology is associated with cognitive impairment in diseases such as type 1 diabetes; however, how capillary flow is affected and the underlying mechanisms remain elusive. Here we show that capillaries in the diabetic mouse brain in both sexes are prone to stalling, with blocks consisting primarily of erythrocytes in branches off ascending venules. Screening for circulating inflammatory cytokines revealed persistently high levels of interleukin-10 (IL-10) in diabetic mice. Contrary to expectation, stimulating IL-10 signalling increased capillary obstruction, whereas inhibiting IL-10 receptors with neutralizing antibodies or endothelial specific knockdown in diabetic mice reversed these impairments. Chronic treatment of diabetic mice with IL-10 receptor neutralizing antibodies improved cerebral blood flow, increased capillary flux and diameter, downregulated haemostasis and cell adhesion-related gene expression, and reversed cognitive deficits. These data suggest that IL-10 signalling has an unexpected pathogenic role in cerebral microcirculatory defects and cognitive impairment associated with type 1 diabetes.
Subject terms: Encephalopathy, Diabetes complications, Type 1 diabetes
Interleukin-10 promotes the formation of microcirculatory defects in the brain associated with cognitive impairment in a mouse model of type 1 diabetes.
Main
The brain is metabolically demanding but lacks considerable stores of energy1. Consequently, it is heavily dependent on an uninterrupted supply of oxygen and nutrients from cerebrovascular networks that finely regulate blood flow to meet changes in metabolic demand2–4. Capillaries have a crucial role in nourishing the brain as they represent more than 95% of the vessel length density5–7; their narrow, low-pressure features maximize the surface area and contact time of blood constituents for oxygen and nutrient exchange8. However, the double-edged sword of these features is they also make capillaries especially prone to short-lasting and long-lasting blood flow stalls. Imaging studies revealed that at any given moment, 0.1–2% of capillaries in the healthy adult mouse brain are stalled9–11. Therefore, it is not surprising that the incidence and duration of these stalling events dramatically increases with disease states such as stroke, Alzheimer’s disease (AD), cytokine storms or lipopolysaccharide treatment12–14. Importantly, therapies targeting one of the root causes of these stalls, namely adherent neutrophils, leads to improved recovery after stroke or restores cognitive function in mouse models of dementia9,12,13.
Type 1 diabetes mellitus (T1DM) is a prevalent chronic disease that produces vascular complications in the eye, heart and kidney. In the brain, these complications manifest with increased incidence of stroke and greater risk for cognitive impairment and dementia14,15. Given these neurological complications, it is surprising that we do not have a better understanding of the aetiology and nature of microcirculatory perturbations in the diabetic brain. Indeed, most work has focused on diabetes-related changes in regional blood flow, the myogenic tone of large cerebral arteries, blood–brain barrier (BBB) permeability or susceptibility to damage after large vessel occlusion16–19. Therefore, we sought to understand microcirculatory disturbances in a mouse model of T1DM and what mechanisms could underlie these defects. We discovered that mice with T1DM had higher rates of stalled capillaries in both sexes, which were mostly occluded by erythrocytes rather than leucocytes. Looking for mechanistic clues, we found abnormally high levels of interleukin-10 (IL-10) in the blood serum of diabetic mice. Contrary to the initial expectations that IL-10 would be protective, as reported in other conditions such as infection, stroke or ageing20–22, stimulating IL-10 signalling increased capillary obstructions and stalls in healthy mice whereas blocking IL-10 in diabetic mice helped prevent these microcirculatory disturbances and cognitive impairment. Collectively these findings strengthen the theory that microvascular pathology has a considerable role in cognitive decline and highlight the complex and duplicitous roles that cytokines can have depending on the biological context.
Results
Microcirculation in the diabetic brain is prone to stalling
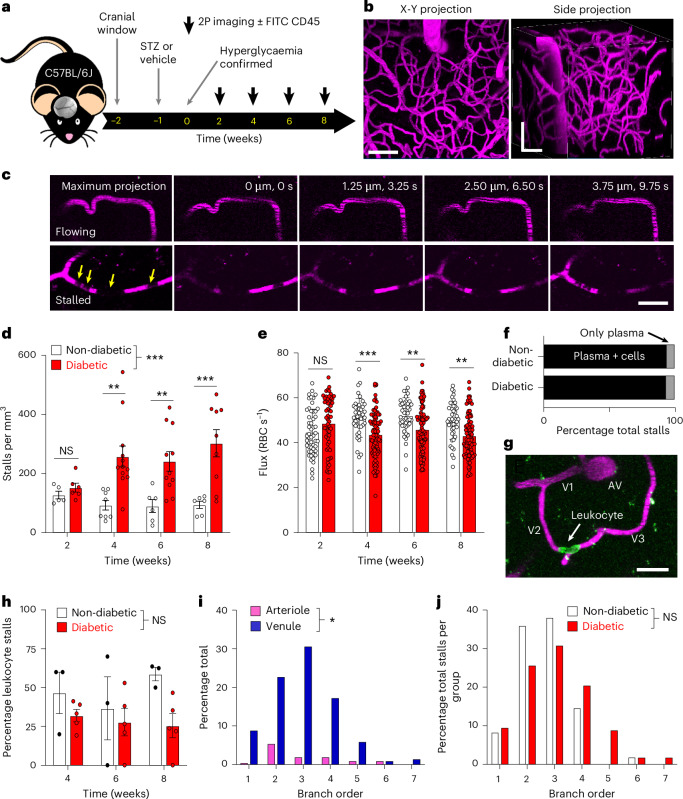
T1DM exacerbates capillary plugging in peripheral organs, such as the heart, kidney and eye23, but whether a similar phenomenon occurs in the brain is unclear. To address this, we performed longitudinal in vivo two-photon imaging of the vasculature in the somatosensory cortex (typical x-y-Z-stack: 489 × 489 × 200 µm) of adult non-diabetic mice or those treated with streptozotocin (STZ) to model T1D (blood glucose greater than 15 mM; Fig. 1a). Under light isoflurane anaesthesia, blood plasma was labelled with intravascular injection of fluorescently tagged 70-kDa dextran and imaged from 2 to 8 weeks after hyperglycaemia was confirmed (Fig. 1a,b). As our z-steps were small enough that multiple images of the same capillary could be discerned over time, flowing capillaries were identified by the presence of red blood cell (RBC) streaks moving through the vessel lumen. Stalled capillaries were defined as those with cells stuck in the vessel (dark gaps denoted by the arrows in Fig. 1c) or those where no streaks were present for at least three image frames (minimum stall duration of 6.5 s; Fig. 1c), similar to previous criteria13. As expected, longitudinal imaging revealed low rates of stalled vessels in healthy mice (Fig. 1d; ~90–127 stalls mm−3). However, diabetes increased the number of stalled capillaries, especially at 4 weeks and beyond (Fig. 1d; ~152–302 stalls mm−3; effect of diabetes F(3,55) = 30.98, P < 0.0001). Consistent with previous work10,13, a subset of capillaries were prone to stalling. For example, capillaries that stalled at 4 weeks had a 21.0% and 28.4% chance of restalling when imaged 2 or 4 weeks later, whereas the chance level of stalling in diabetic mice was approximately 3.08%. Elevated rates of stalling in diabetic mice were also associated with significantly lower levels of RBC flux in cortical capillaries at 4 weeks and beyond, relative to non-diabetic controls (Fig. 1e; effect of diabetes F(1,507) = 19.2, P < 0.0001).
Fig. 1. In vivo imaging shows abnormally high levels of stalled capillaries and lower RBC flux in the diabetic mouse cortex.
a, Experimental timeline of diabetes induction and two-photon imaging. b, Maximal intensity two-photon X-Y and side (X-Z) projections showing the cerebral vasculature (shown in magenta). c, Successive images through a Z-stack show flowing or stalled capillaries. Note the streaking pattern caused by RBC movement in flowing capillaries or absence of streaking in those that are stalled. The yellow arrows indicate unlabelled cells stalled in the capillary. d, Estimated stalls per mm3 in non-diabetic (n = 5, 8, 6 and 6 mice for weeks 2, 4, 6 and 8, respectively) and diabetic mice (n = 6, 12, 11 and 10 mice for weeks 2, 4, 6 and 8, respectively) in the weeks after hyperglycaemia was confirmed. P values for 2, 4, 6 and 8 weeks: 0.9865, 0.0012, 0.0096 and 0.0004, respectively. e, RBC flux (RBC s−1) in non-diabetic (n = 53, 45, 45 and 45 capillaries for weeks 2, 4, 6 and 8, respectively) and diabetic capillaries (n = 57, 90, 90 and 90 capillaries for weeks 2, 4, 6 and 8, respectively) over time. P values for 2, 4, 6 and 8 weeks: 0.1278, 0.0002, 0.0031 and 0.0012, respectively. f, Percentage of stalls with blood cells plugging the capillary or without (plasma + cells or just plasma). g, Representative image showing a CD45.2-labelled leucocyte plugging a second-order branch (V2) from an AV. h, Graph showing the percentage of leucocyte-stalled capillaries in non-diabetic and diabetic groups (n = 3 and 5 mice, respectively) at different time points. Note that leucocytes were not imaged at the 2-week time point. i, Percentage of stalled capillaries as related to the branch order of the PA or AV. j, Percentage of stalled capillaries as a function of branch order in each experimental group. Data in d,e,h were analysed with a two-way ANOVA and Šidák’s multiple-comparisons test. Data in I,j were analysed with a two-sided Mann–Whitney U-test. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001. b, Scale bar, 50 µm. c,g, Scale bar, 20 µm. Data are expressed as the mean ± s.e.m.
To further understand stalls, we first classified them based on their appearance, that is, whether they exhibited cells or not. The overwhelming majority of stalls in both experimental groups (93–94%) were those with cells plugging the capillary, whereas a small fraction did not appear to have any cells in the lumen nor any evidence of streaking (termed ‘only plasma’ in Fig. 1f). To determine the predominant cell type plugging the capillaries (that is, RBCs or leucocytes), a subset of mice were imaged at 4, 6 and 8 weeks, and injected with fluorescein isothiocyanate (FITC)-conjugated anti-CD45.2 antibodies to label circulating leucocytes24. As shown in Fig. 1g, leucocytes were easily visualized in stalled capillaries. While previous studies identified leucocytes as the primary cause of stalls in AD or stroke12,13, our results indicate that only 25–32% of total stalls in diabetic mice were associated with a leucocyte (Fig. 1h). Our analysis revealed that the fraction of leucocyte-stalled capillaries did not differ significantly between non-diabetic controls and diabetic groups, nor did it change over time (Fig. 1h; effect of diabetes F(1,6) = 3.90, P = 0.10; effect of time F(1.06,6.37) = 3.90, P = 0.46). Furthermore, leucocyte-stalled capillaries were just as vulnerable to restalling at later imaging time points as RBC-stalled capillaries (leucocytes had a 25% chance of restalling versus 24.5% chance with RBC-based stalls). Lastly, to understand what parts of the vascular tree were most susceptible, we examined where stalls occurred as a function of branch order from the penetrating arteriole (PA) or ascending venule (AV) (see the branch orders in Fig. 1g). Most stalls occurred in capillary branches proximal to the AV (V2–4, Fig. 1i), which did not differ significantly between diabetic and non-diabetic mice (Fig. 1j). These results show that diabetic mice are prone to stalled capillaries blocked mostly by presumptive RBCs, especially in capillary branches proximal to the AV.
Effect of insulin treatment and sex on capillary obstruction
To extend our in vivo observations and improve our understanding of how sex and insulin treatment affect capillary stalling in several brain regions, we injected 5-µm diameter fluorescent microspheres intravenously into male and female diabetic mice treated with or without insulin and extracted their brain 3 days after injection (Fig. 2a). Using the microsphere assay allowed us to titre the density of microsphere-obstructed capillaries to a level that was comparable to our in vivo estimates of stalled capillaries (~30–150 microspheres mm−3 versus 90–300 stalls mm−3). Previous work from our laboratory and others has shown that these small microspheres do not cause BBB disruption, local recruitment of microglia or microinfarcts, which are commonly found after injection of larger emboli (>10-µm diameter)5,10,25. Furthermore, as the dose of microspheres can be carefully controlled between mice, it allows high-throughput screening of obstructed capillary density across many experimental groups and different brain regions not easily accessible with in vivo microscopy5,10. As anticipated, STZ treatment led to a significant increase in blood glucose levels in the uncontrolled (that is, not treated with insulin) diabetic group (F(1,114) = 176.1, P < 0.0001), whereas insulin treatment lowered blood glucose but not to completely normal levels (F(1,65) = 32.8, P < 0.0001; compare ‘diabetic’ and ‘diabetic + insulin’ to ‘non-diabetic’ groups in Fig. 2b). Relative to non-diabetic control mice, body weights were lower for both uncontrolled (F(1,114) = 17.55, P < 0.0001) and insulin-treated diabetic mice (F(1,65) = 10.21, P < 0.01), although this was not statistically significant at any specific time point (Fig. 2c). Consistent with our in vivo findings on capillary stalling, we found a significant increase in the number of microsphere-obstructed capillaries in the forebrain of diabetic mice when collapsed across sex (Fig. 2d). We should note that microsphere densities were similarly elevated in diabetic mice regardless of whether they received two or three injections of STZ (t(16) = 0.05, P = 0.41). Insulin treatment did not significantly lower the density of obstructions across the forebrain from that found in uncontrolled diabetic mice (Fig. 2d). Consistent with a recent study26, plotting blood glucose values in all groups as a function of obstruction density revealed a significant positive linear relationship (Fig. 2e; R2 = 0.52, P < 0.0001). Based on previous work showing that there are significant regional differences in susceptibility to capillary stalling5, we also quantified microspheres in different brain regions. This analysis indicated that capillary obstructions were significantly greater in uncontrolled diabetic mice relative to non-diabetic controls (Fig. 2f; effect of diabetes F(1,373) = 59.1, P < 0.0001), which also varied significantly according to brain region (effect of region F(12,373) = 20.31, P < 0.0001; diabetes × region interaction F(12,373) = 1.7, P = 0.06), with the highest density in the retrosplenial cortex. Insulin-treated diabetic mice showed a slight but significant reduction in obstruction density compared to uncontrolled diabetic mice (Fig. 2f; effect of insulin F(1,244) = 4.39, P = 0.04). Lastly, we stratified our obstruction density analysis according to sex and discovered that there were no sex differences in either non-diabetic or diabetic mice (Fig. 2g; effect of sex in non-diabetic mice F(1,181) = 0.66, P = 0.42 and diabetic mice F(1,165) = 0.25, P = 0.62). These results indicate that increased capillary obstructions in diabetic mice are only partially affected by insulin treatment and are not dependent on sex.
Fig. 2. Effect of insulin treatment and sex on capillary obstruction.
a, Top, Timeline of diabetes induction and microsphere injection. Bottom, Representative widefield images showing fluorescent microspheres (green) plugging the lumen of capillaries filled with fluorescent dextran (magenta) in the somatosensory cortex. b, Weekly blood glucose levels in the experimental groups (non-diabetic, n = 7; diabetic, n = 14; diabetic + insulin, n = 6 mice). ***P < 0.001, ****P < 0.0001. c, Weekly body weight measurements in the experimental groups (non-diabetic, n = 7; diabetic, n = 14; diabetic + insulin, n = 6 mice). d, Quantification of microsphere-plugged capillaries across the forebrain in non-diabetic, uncontrolled diabetic and insulin-treated mice (n = 16, 15 and 6 mice, respectively; **P = 0.0013, NS P = 0.55). e, Regression analysis showing a significant relationship between blood glucose levels and density of microsphere-plugged capillaries in the mouse forebrain (n = 37 mice). f, Density of microsphere-plugged capillaries across different brain regions in each experimental group (*P = 0.037, ****P < 0.0001). g, Regional density of microsphere-plugged capillaries as a function of sex (non-diabetic, n = 8 male and 8 female mice; diabetic, n = 7 male and 8 female mice). CC, corpus callosum; FrA, frontal association cortex; GIC, granular or dysgranular insular cortex; HPC, hippocampus; Hyp, hypothalamus; motor, primary or secondary motor cortex; PRh, peri-rhinal cortex; PrL, prelimbic cortex; RS, retrosplenial cortex; S1FL, primary forelimb somatosensory cortex; Thal, thalamus; V1, primary visual cortex. Data in b,c,f,g were analysed using a two-way ANOVA followed by Tukey’s multiple-comparisons test when appropriate. Data in d were analysed using a two-tailed unpaired t-test. Data in e were analysed with a two-sided linear regression. a, Scale bars from left to right: 1 mm, 50 µm and 20 µm. Data are expressed as the mean ± s.e.m.
High levels of IL-10 in diabetic blood is associated with obstructed capillaries
To elucidate the mechanisms underlying the increased stalling rates, we screened for multiple cytokines and chemokines in blood serum samples collected after 8 weeks of uncontrolled diabetes. Multiplex analysis revealed that levels of certain cytokines, including IL-1β, IL-2, IL-6, IL-10, interferon gamma-induced protein (IP-10), tumour necrosis factor (TNFα), keratinocyte chemoattractant/chemokine (C-X-C motif) ligand 1 (KC/CXCL1), macrophage inflammatory protein-1 alpha (MIP-1α), MIP-2, MIP-3 and MCP-1, were significantly higher in diabetic mice than in non-diabetic controls (Fig. 3a; for raw values see Extended Data Fig. 1a). Given that diabetic mice showed significantly higher rates of capillary stalling well before 8 weeks (Fig. 1d), we re-examined the cytokines IL-1β, IL-6, IL-10, monocyte chemoattractant protein-1/chemokine (CC-motif) ligand 2 (MCP-1/CCL2)), KC (CXCL1), IL-17 and MIG1 (CXCL9) at 4 weeks. Among the cytokines re-examined, only IL-10 was significantly elevated at both 4 and 8 weeks relative to non-diabetic mice (Fig. 3b and Extended Data Fig. 1b–e). The levels of IL-1β and IL-6 were very low at the 4-week time point and below the detection limits. To determine if IL-10 was elevated in other animal models of T1D, we examined blood serum cytokine levels in hyperglycaemic non-obese diabetic (NOD) mice (n = 10 mice; blood glucose 28.8 ± 4.0 mM) and their respective controls (n = 13 mice, blood glucose 8.75 ± 1.2 mM). Our analysis showed that IL-10 was significantly elevated in NOD mice relative to controls (Extended Data Fig. 1f). The finding that IL-10 levels were elevated in diabetic mice was somewhat surprising given that IL-10 is generally considered an anti-inflammatory cytokine. However, reports have shown that IL-10 can have pleiotropic effects and function both as a pro-inflammatory and an anti-inflammatory cytokine27. To further understand the consequence of elevated IL-10, we injected non-diabetic mice with IL-10 or control albumin protein (0.5 µg, intravenously) and quantified microsphere obstruction density (Fig. 3c). Our results show that injecting IL-10 intravenously can significantly increase capillary obstructions (Fig. 3d). To rule out the possibility that albumin (used in control mice) could affect capillary plugging independently, we compared microsphere density in mice injected with saline or 0.5 µg albumin mixed in saline, and found no differences (t(7) = 0.34, P = 0.75). These results suggest that circulating IL-10 could have a role in capillary stalling.
Fig. 3. Elevated levels of IL-10 in diabetic blood is associated with capillary plugging.
a, Multiplex immunoassay showing normalized expression of cytokines and chemokines in the blood serum of non-diabetic (n = 12) and diabetic (n = 10 mice) mice collected 8 weeks after confirmation of hyperglycaemia. *P < 0.05, **P < 0.01, ***P < 0.001. b, IL-10 levels were elevated in diabetic blood serum 4 weeks after confirmation of hyperglycaemia (non-diabetic = 12, diabetic = 12 mice; *P = 0.031). c, Experimental timeline to examine the effect of intravenous injection of IL-10 or control albumin protein on capillary plugging. d, Normalized density of microsphere-plugged capillaries in the forebrain of control and IL-10-injected mice (n = 8 and 6 mice, respectively; ***P = 0.0005). Data in a,b,d were analysed using a two-tailed unpaired t-test. Data are expressed as the mean ± s.e.m.
Extended Data Fig. 1. Blood serum cytokine levels in non-diabetic and diabetic mice.
a, Concentration for each cytokine/chemokine (pg/mL) in blood serum of non-diabetic (black dots) and STZ induced diabetic mice (red dots) at 8 weeks. b-e, Graphs show expression of CCL2/MCP-1 (b), CXCL1/KC (c), IL-17A (d), and CXCL9/MIG (e) in blood serum 4 weeks after confirmation of hyperglycemia (n=8 mice/group). f, Expression of blood serum cytokines in non-obese diabetic (NOD, n=10 mice) mice normalized to non-diabetic controls (NOR, n = 13 mice). Data in b-f analysed by unpaired two-tailed t-tests. Data are expressed as mean ± SEM. *p<0.05.
Endothelial IL-10 receptor knockdown alleviates capillary obstructions
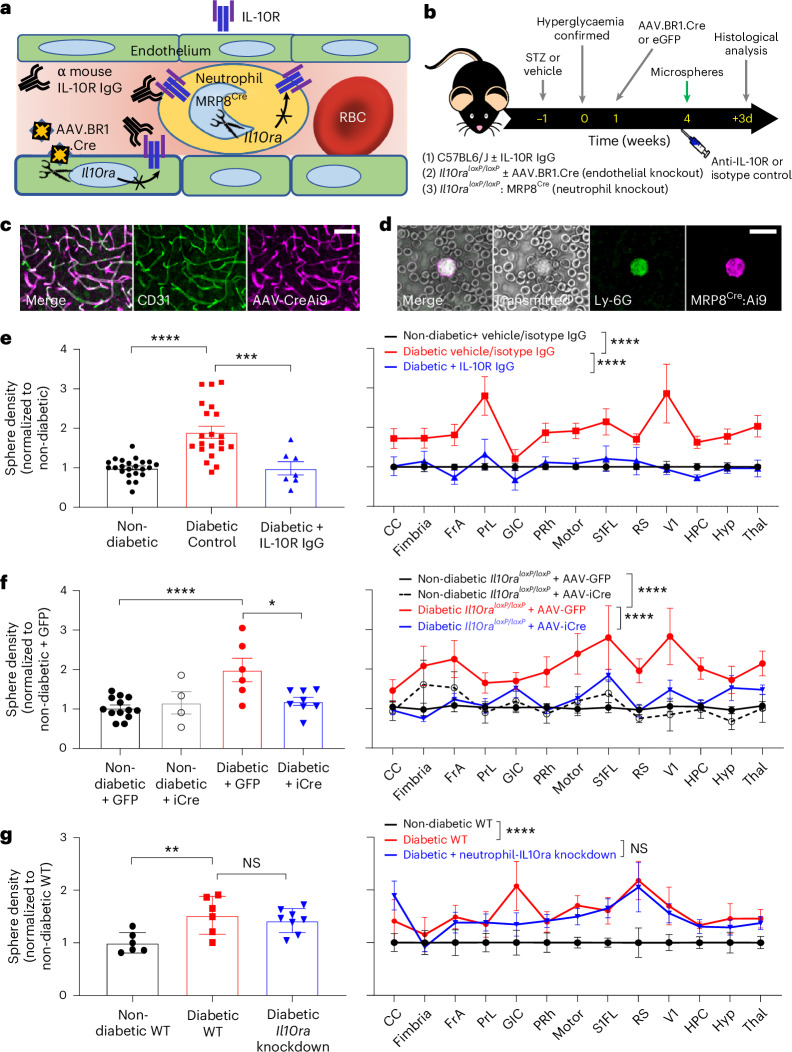
Given that high levels of circulating IL-10 were associated with stalled capillaries and that IL-10 receptors are expressed on both endothelial cells (Extended Data Fig. 2a,b) and neutrophils, we next explored whether broad-spectrum inhibition of IL-10 receptors, or cell-specific knockdown in vascular endothelial cells or neutrophils, could prevent capillary obstructions in diabetic mice (Fig. 4a,b). To knock down IL-10 receptors in endothelial cells, diabetic Il10raloxP/loxP mice were injected intravenously with AAV-BR1-iCre that selectively targets brain endothelial cells28 or control vector (AAV-BR1-eGFP) 3 weeks before microsphere injection. For neutrophil-specific knockdown, we crossed Mrp8Cre mice with Il10raloxP/loxP mice (Extended Data Fig. 2c). Cre recombinase activity in either brain endothelial cells or neutrophils was confirmed in the Cre-dependent tdTomato reporter mouse (Ai9; Fig. 4c,d). Furthermore, we verified Il10ra knockdown after AAV-BR1-iCre injection from isolated endothelial cells using quantitative PCR (qPCR) or PCR for Mrp8Cre:Il10raloxP/loxP mice (Extended Data Fig. 2b,c).
Extended Data Fig. 2. Validation of IL10ra knockdown in endothelial cells and neutrophils.
a, Isolated endothelial cells (n=2 mice/group) showing enriched expression of genes typically expressed in endothelium (Vegfr2, Tie2, Cd31, Cdh5), with very low levels of gene expression associated with astrocytes (Gfap), neurons (NeuN), leukocytes (Cd45) or microglia (Tmem119). b, Box plot shows median, upper/lower quartile and max/min values from qPCR data showing loss of Exon 3 IL10ra gene expression in endothelial cells isolated from Il10ra floxed mice injected with AAV-BR1-iCRE (n=4 mice), relative to controls (WT: Wild type; Il10ra flox/flox: Il10ra flox/flox mice; WT mice injected with AAV-BR1-iCRE). c, Breeding strategy and representative PCR results (reproduced >5 times) for Mrp8cre:IL10ra floxed mice.
Fig. 4. IL-10 receptor inhibition with neutralizing antibodies or endothelial cell-specific knockdown alleviates capillary plugging in diabetic mice.
a, Illustration showing how IL-10R signalling could impact capillary obstructions through vascular endothelial cells or neutrophils and the therapeutic approaches tested. b, Experimental timeline and three treatment groups to inhibit IL-10R signalling: (1) injection of IL-10R neutralizing antibodies in C57BL/6J wild-type (WT) mice; (2) injection of AAV.BR1.iCre or control virus (AAV.BR1.eGFP) in Il10raloxP/loxP mice for endothelial cell-specific knockdown of Il10ra receptors; (3) neutrophil-specific knockdown using Il10raloxP/loxP mice crossed with MRP8Cre mice. c,d, Representative images showing validation of Cre recombinase activity in either brain endothelial cells using CD31 immunolabelling (c) or neutrophils using Ly-6G antibody (d) with Cre-dependent tdTomato reporter mouse (Ai9). e, Effect of IL-10R neutralizing antibody treatment on the density of microsphere-plugged capillaries in non-diabetic controls, diabetic controls or diabetic + IL-10R IgG-treated mice (n = 23, 21 and 7 mice, respectively). Microsphere densities are shown collapsed across all forebrain regions (left, ***P = 0.0004, ****P < 0.0001) or in specific brain regions (right, ****P < 0.0001). f, Effect of endothelial cell-specific Il10ra knockdown on density of plugged capillaries across the forebrain (left, *P = 0.011, ****P < 0.0001) or in specific brain regions (right, ****P < 0.0001) in non-diabetic (AAV-GFP: n = 13 mice; AAV-iCre: n = 4 mice) and diabetic mice injected with AAV-GFP (n = 6 mice) or AAV-iCre (n = 8 mice). g, Density of microsphere-plugged capillaries in the forebrain (left, NS P = 0.76, **P = 0.009) or specific brain regions (right, NS P = 0.33, ****P < 0.0001) in non-diabetic WT (n = 6 mice), diabetic WT (n = 6 mice) or diabetic Il10ra knockdown in neutrophils (diabetic IL-10ra knockdown, n = 8 mice). Data in e,f were analysed using a two-tailed unpaired t-test (left) or a two-way ANOVA followed by Šidák’s multiple-comparisons test (right). c,d, Scale bar, 20 µm. Data are expressed as the mean ± s.e.m.
To broadly inhibit IL-10 signalling, we treated hyperglycaemic diabetic mice with intravenous injection of IL-10R neutralizing antibody or isotype control antibody. Mice were injected with microspheres and the density of obstructed capillaries was quantified 3 days later (see the timeline in Fig. 4b). Diabetic mice treated with the IL-10R neutralizing antibody had significantly lower density of obstructed capillaries in the forebrain or across different brain regions relative to isotype-control-treated diabetic mice (Fig. 4e; F(1,335) = 32.83, P < 0.0001). Similarly, treating diabetic mice with IL-10 neutralizing antibody significantly reduced the density of microsphere-plugged capillaries in the forebrain (Extended Data Fig. 3a,b). We next examined the effect of AAV-mediated knockdown of endothelial cell IL-10 signalling. Diabetic Il10raloxP/loxP mice injected with AAV-BR1-iCre exhibited a significant reduction in the density of obstructed capillaries compared to diabetic mice treated with control AAV-BR1-eGFP (Fig. 4f). This reduction was evident for the forebrain analysis and across different brain regions (Fig. 4f; F(1,156) = 48.10, P < 0.0001). We next examined the density of obstructed capillaries in diabetic mice with neutrophil-specific knockdown of IL-10 receptors or Cre− (‘WT’) controls (Fig. 4g). Our analysis revealed no significant differences between diabetic treatment groups (Fig. 4g; F(1,156) = 0.95, P = 0.33). Importantly, none of the manipulations described above significantly altered blood glucose levels in diabetic mice, thereby arguing against the possibility that the rescue effects of IL-10R antibodies or Il10ra knockdown were directly caused by lowering blood glucose levels (Extended Data Fig. 4; F(5,53) = 0.41, P = 0.83). In summary, these findings show that neutralizing antibodies or endothelial cell-specific inhibition of IL-10 receptor signalling alleviate capillary obstructions in diabetic mice.
Extended Data Fig. 3. Treating diabetic mice with IL-10 neutralizing antibody reduced the density of microsphere plugged capillaries in the brain.
a, Experimental timeline. b, Density of microsphere plugged capillaries in the forebrain in diabetic mice treated with isotype control antibody or IL-10 neutralizing antibody, normalized to non-diabetic controls (*p=0.015, **p=0.009; n=8, 9 and 8 mice per group). Data in b analysed with two-tailed unpaired t-tests. Data are expressed as mean ± SEM.
Extended Data Fig. 4. Blood glucose levels in diabetic mice at 4 weeks across different treatment groups.

Blood glucose levels in diabetic mice at 4 weeks. One-way ANOVA indicated no significant differences between diabetic groups (F(5,38)=0.53, p=0.75; n=21, 7, 6, 8, 9 and 8 mice, respectively). Data are expressed as mean ± SEM.
Neutralizing IL-10 signalling alleviates capillary stalling in vivo and improves cerebral blood flow
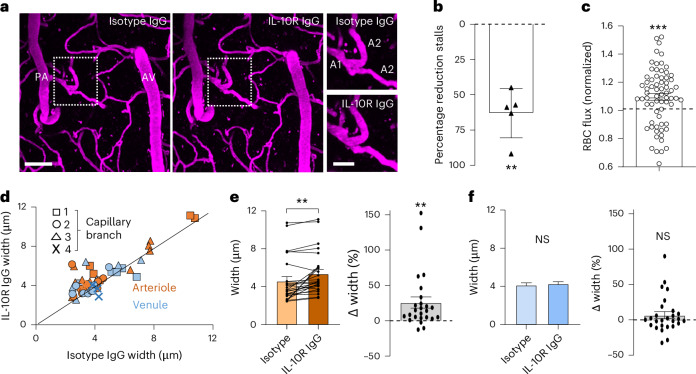
As treating diabetic mice with a neutralizing antibody represents the most clinically translatable approach for modulating IL-10 signalling in vivo, we next imaged the cerebral vasculature in diabetic mice after injection of isotype control antibody and then again 1 week later after treatment with IL-10R neutralizing antibody (Fig. 5a). Two-photon imaging of the cortical microcirculation revealed a 63% decrease in the number of stalled capillaries in IL-10R-antibody-treated mice (Fig. 5b). Similarly, IL-10R neutralizing antibody treatment significantly increased RBC flux in capillaries relative to isotype controls (Fig. 5c). To better understand how the treatment was affecting the microcirculation, we measured the width of capillaries across the proximal branches (branch orders 1–4) from the PA or AV (see the examples in Fig. 5a). Our analysis indicated wider capillaries from proximal branches off the PA after IL-10R neutralizing antibody treatment (Fig. 5d). By collapsing the width measurements across first to fourth order capillary branches on the arteriole or venule side, we found a significant increase in the diameter of capillaries branching off the PA after IL-10R neutralizing antibody treatment (Fig. 5e), whereas those on the venule side did not change significantly (Fig. 5f). These results indicate that IL-10R neutralizing antibody treatment reduces the fraction of stalled capillaries, increases capillary flux and dilates proximal capillary branches coming off the PA.
Fig. 5. Treating diabetic mice with IL-10R neutralizing antibody lowers stalling rates, and increases capillary flux and capillary width.
a, Representative in vivo two-photon Z-projection images showing the fluorescently labelled vasculature (shown in magenta) in the diabetic somatosensory cortex after injection of isotype control antibody and again after treatment with IL-10R neutralizing antibody 1 week later. The insets on the right show lower-order capillary branches off the PA in each treatment condition. b, Percentage reduction in stalling density in diabetic mice treated with IL-10R neutralizing antibody relative to isotype controls (n = 5 mice imaged after both treatments). P = 0.009 c, RBC flux in capillaries from diabetic mice treated with IL-10R neutralizing antibody normalized to the diabetic isotype controls (n = 69 capillaries from five mice). d, Unity plot showing the width of the proximal capillary branches (branches 1–4) off the PA (brown, n = 25 capillaries from four mice) or AV (blue, n = 25 capillaries from four mice) after isotype control or IL-10R neutralizing antibody treatment. e, Absolute or percentage change in width of the capillaries branching off the PA (brown) (n = 25 capillaries from four mice). f, Absolute or percentage change in width of capillaries branching off the AV (n = 27 capillaries from four mice). Data in e,f (left) were analysed with a two-tailed paired t-test. Data b,c,e,f (right) were analysed with a two-sided one-sample t-test. **P value in b: 0.009; P values in e (left) 0.001 and e (right) 0.003; ***P = 0.0002. a, Scale bar, 50 µm (inset, 20 µm). Data are expressed as the mean ± s.e.m.
Next, we examined whether chronic treatment of diabetic mice with IL-10R neutralizing antibodies could improve stimulus-evoked vascular responses (Fig. 6a). We used laser Doppler flowmetry to assess cerebral blood flow (CBF) in urethane-anaesthetized mice before and after 5% CO2 challenge, vibrotactile limb stimulation or exposure to isoflurane. Our findings show that the normal increase in CBF after CO2 exposure was blunted in isotype-treated diabetic mice, whereas treatment with IL-10R neutralizing antibody helped to partially normalize CBF responses (Fig. 6b–e). Similarly, CBF responses to sensory limb stimulation were lower in isotype-treated diabetic mice relative to non-diabetic mice (Fig. 6f–i). Treatment with IL-10R neutralizing antibody produced a small but significant increase in CBF responses, primarily near the end of the vascular response (7.5–15 s after stimulation; Fig. 6f–i). Interestingly, no significant differences were observed among experimental groups in response to isoflurane (Fig. 6j–m). This latter finding helps rule out the possibility that elevated stalling rates in diabetic mice were related to isoflurane during two-photon imaging. To determine if diabetes or IL-10R neutralizing antibody affected general cardiovascular function, we assessed oxygen saturation and heart rate. There were no significant differences between experimental groups in these parameters (Extended Data Fig. 5a,b; O2 saturation F(2,17) = 0.04, P = 0.96; hazard ratio (HR) F(2,17) = 0.03, P = 0.97).
Fig. 6. Effect of IL-10R neutralizing antibody on CBF in diabetic mice.
a, Schematic showing the timeline of the experimental procedures and the CBF measurements using laser Doppler flowmetry. CBF measurements included peak amplitude, time to peak and area under the curve (a.u.c.). b, Normalized blood flow changes in response to inhalation of 5% CO2. c–e, Peak amplitude (c), time to peak (d) and a.u.c. (e) in non-diabetic or diabetic mice treated with isotype control or IL-10R neutralizing antibody (n = 12, 11 and 16 mice, respectively). Significance in c: **P = 0.005 and 0.0002; significance in e: **P = 0.005 and 0.006. f, Normalized blood flow changes in response to 100-Hz vibrotactile stimulation of the limb. g–i, Peak amplitude (g), time to peak (h) and a.u.c. (i) in the three groups (non-diabetic, n = 11; diabetic + isotype, n = 11; diabetic + IL-10R IgG, n = 16 mice). Significance in g: *P = 0.030 and 0.024; significance in h: *P = 0.031 and 0.013; significance in i: *P = 0.011, **P = 0.006. j, Normalized blood flow changes in response to 1% isoflurane mixed in air. k–m, Peak amplitude (k), time to peak (l) and a.u.c. (m) of blood flow changes in the three groups (non-diabetic, n = 12; diabetic + isotype, n = 10; diabetic + IL-10R IgG, n = 16 mice). The grey areas in b,f,j indicate the duration of the stimuli used to evoke changes in blood flow (60, 10 and 30 s, respectively). Data in c–e,g–i,k–m were analysed using a two-tailed unpaired t-test. Data are expressed as the mean ± s.e.m.
Extended Data Fig. 5. Assessment of cardiovascular function during laser Doppler experiments.
There were no significant differences in oxygen saturation (a) or heart rate (b, beats per minute) between non-diabetic and diabetic mice treated with isotype or IL-10R neutralizing antibody (n = 7, 7 and 6 mice per group). Data in a and b were analysed with 1-way ANOVA. Data are expressed as mean ± SEM.
IL-10R neutralizing antibody treatment improves cognitive function
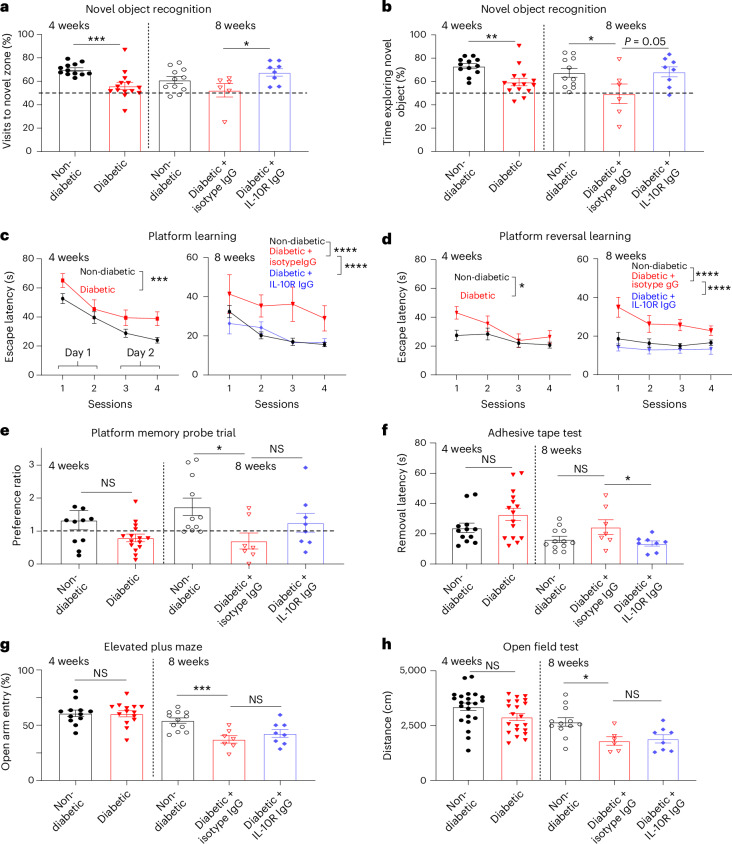
Growing evidence suggests that vascular pathology has a critical role in cognitive impairment29,30. Therefore, we tested whether chronic treatment with IL-10R neutralizing antibody could ameliorate deficits in cognitive and sensorimotor functions (Fig. 6a). First we used the novel object recognition (NOR), a common test of memory. This test showed that diabetes-related impairments in the frequency of visits to a novel zone (Fig. 7a) or time spent exploring the novel object (Fig. 7b) can be reversed in diabetic mice given IL-10R neutralizing antibody. We next assessed spatial learning and memory in the Morris water maze. At both 4 and 8 weeks, control-treated diabetic mice took significantly longer to find the submerged platform during the initial platform learning trials (Fig. 7c), and after the platform location was switched to a new location (‘reversal learning’; Fig. 7d). In both tests, performance improved in diabetic mice treated with IL-10R neutralizing antibody given that they took significantly less time to find the platform relative to control-treated diabetic mice (Fig. 7c,d). Performance in the memory probe trial was marginally worse in diabetic mice; IL-10R neutralizing antibody treatment slightly, but not significantly, increased this metric (Fig. 7e). Group differences in learning and memory cannot be explained by differences in swim speed or gross visual abilities (based on the visible platform test) because no significant differences were found across experimental groups (Extended Data Fig. 6a,b). To determine if sensorimotor behaviours were affected, we examined performance in the adhesive tape removal test and open field activity. Control-treated diabetic mice exhibited significantly longer latencies to remove the tape relative to IL-10R neutralizing antibody-treated mice (Fig. 7f). In the elevated plus maze, anxiety-like behaviour in diabetic mice reflected by the lower preference for entry into the open arms, was not affected by IL-10R treatment (Fig. 7g), while diabetic mice showed reduced activity in the open field, particularly at week 8; inhibiting IL-10R had no effect on this behaviour (Fig. 7h). Behavioural changes were not simply a function of altering blood glucose as a two-way analysis of variance (ANOVA) did not reveal any significant differences in blood glucose based on IL-10R neutralizing IgG treatment (P = 0.21), time (P = 0.74) or treatment × time interaction (P = 0.62). These results show that IL-10R receptor inhibition in diabetic mice generally improves metrics of cognitive and somatosensory and motor function (NOR, water maze and tape tests), but has minimal or no effect on general ambulatory or anxiety-like behaviours.
Fig. 7. Long-term IL-10 receptor inhibition improves cognitive function in diabetic mice.
a,b, Graphs showing the frequency of visits to the novel object zone (a, *P = 0.027, ***P = 0.0008) or percentage time spent exploring a novel object (b, *P = 0.040, **P = 0.002) at 4 weeks in non-diabetic or diabetic mice (n = 12 and 14 mice, respectively), or at 8 weeks in non-diabetic or diabetic mice treated with isotype control or IL-10R neutralizing antibody (n = 11, 6 and 8 mice, respectively). c, Escape latency for learning the hidden platform location in the Morris water maze at 4 weeks in non-diabetic and diabetic mice (***P = 0.0004; n = 26 and 23 mice, respectively) or at 8 weeks (****P < 0.0001; n = 12, 7 and 8 mice for non-diabetic, diabetic + isotype and diabetic + IL-10R neutralizing antibody, respectively). d, Escape latency for learning the new platform location in the water maze (‘platform reversal learning’) in non-diabetic and diabetic mice at 4 (*P = 0.01; n = 12 and 15 mice, respectively) and 8 (****P < 0.0001; n = 12, 7 and 8 mice for non-diabetic, diabetic + isotype and diabetic + IL-10R neutralizing antibody, respectively) weeks. e, Preference for quadrant with hidden platform (‘platform memory probe trial’) in non-diabetic and diabetic mice at 4 (NS P = 0.07; n = 11 and 15 mice, respectively) and 8 (NS P = 0.171, *P = 0.015; n = 11, 7 and 8 mice for non-diabetic, diabetic + isotype and diabetic + IL-10R neutralizing antibody, respectively) weeks. f, Graph showing latency of tape removal (s) in different experimental groups at 4 (NS P = 0.115; n = 12 and 15 mice) and 8 (NS P = 0.093, *P = 0.047; n = 12, 7 and 8 mice) weeks. g, Percentage of time spent in the open arms of the elevated plus maze at 4 (NS P = 0.92; n = 12 and 14 mice) and 8 (NS P = 0.92; ***P = 0.0007; n = 12, 7 and 8 mice) weeks. h, Distance travelled by mice in each experimental group in the open field test at 4 (NS P = 0.057; n = 22 and 21 mice) and 8 (NS P = 0.734, *P = 0.013; n = 12, 6 and 8 mice) weeks. In a,b,e–h, a two-tailed unpaired t-test was used to analyse the data at each time point. Data in c,d were analysed using a two-way ANOVA followed by Tukey’s multiple-comparisons test. Data are expressed as the mean ± s.e.m.
Extended Data Fig. 6. Effect of diabetes on swim speed and visibility of mice.
a, Swim Speed in Morris water maze at 4 and 8 week testing periods (n=11, 15, 12, 7 and 8 mice, respectively). b, Escape latencies in the visible platform test at 8 week testing period (n=11, 7 and 7 mice, respectively). Data analysed with two-way ANOVA (a) and unpaired two-tailed t-tests (b). ns: not significant. Data are expressed as mean ± SEM.
Transcriptional changes in brain endothelium after IL-10R antibody treatment
To further understand how diabetes and IL-10R neutralizing antibody affect vascular endothelial gene expression, we treated diabetic mice with IL-10R neutralizing or isotype control antibody (Fig. 6a) and extracted endothelial cells from the brain. For a complete list of all significant differentially expressed genes (DEGs) comparing diabetic to non-diabetic or diabetic IL-10R IgG-treated mice to isotype controls, see Supplementary Data 1 and 2. As expected when compared to non-diabetic mice, endothelial gene expression from diabetic mice (isotype-treated) was enriched in those associated with metabolic processes, immune and viral responses, NOD receptor-like signalling pathways (Irf7, Oas1a, Stat1, Irf9, Mx2; Extended Data Fig. 7a–d). However, when comparing diabetic mice treated with IL-10R versus isotype control antibody, we discovered differential expression of genes involved in cell responses to stress or damage (Cdkn1a), cytoskeletal architecture (Akap12, Septin10) and tumorigenesis (Sox12, Cdk5rap3) (Fig. 8a,b). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis showed that IL-10R antibody treatment upregulated gene pathways involved in the regulation of reactive oxygen species and energy metabolism, and downregulated gene pathways that regulate cellular adhesion, platelet activation and atherosclerosis (terms underlined in Fig. 8c). In particular, focal adhesion-related and platelet activation-related genes that were significantly downregulated include Col4a1, Parvb, Rapgef1, Rasgrp2, Septin11, Ppp1r12a, Vcl, Fyn, Lama3, Pcdh12 and Pla2g4b (Fig. 8b,d). Of note, these genes are also significantly altered 2 days after stroke31 when poor capillary flow and stalling is a major issue. These results show that IL-10R neutralizing antibody treatment downregulates the signalling pathways that could potentially regulate capillary stalling, such as those associated with haemostasis and cell adhesion.
Extended Data Fig. 7. Differentially expressed genes in the brain endothelium of diabetic mice relative to non-diabetic controls.
a, GO analysis shows the 10 most significant (two sided, p-values adjusted for multiple comparisons) downregulated (left) or upregulated (right) genes related to biological processes (BP), cellular component (CC) or molecular function (MF). b, KEGG analysis shows the top 20 down-regulated (left) or up-regulated (right) pathways in diabetic mice relative to non-diabetic controls. c, Volcano plots show gene expression in diabetic isotype treated mice relative to non-diabetic controls (n=6 mice per group), based on log2 fold change and statistical significance (adjusted p value). d, Heat map shows scaled gene expression for top 30 most significantly up or down regulated genes in each mouse. Numbers on bottom represent individual mouse IDs.
Fig. 8. IL-10R neutralizing antibody treatment downregulates the genes associated with haemostasis and cell adhesion.
a, Volcano plot showing DEGs as a function of log2 fold change and statistical significance (two-sided P value adjusted for multiple comparisons) between diabetic mice treated with IL-10R neutralizing antibody versus isotype control (n = 6 mice per group). The top ten genes most significantly upregulated or downregulated have been labelled. b, Heatmap showing the scaled expression of the top 30 most significantly upregulated or downregulated DEGs in diabetic IL-10R IgG-treated mice relative to isotype controls (bottom, individual mouse IDs 33–44). c, Dot plot showing KEGG enrichment analysis of DEGs (two-sided test) for the top 20 upregulated (left) or downregulated (right) pathways. d, Histograms showing normalized read counts for genes implicated in regulating cell adhesion and platelet activation, which were significantly downregulated with IL-10R antibody treatment (n = 6 mice per group, all Padj < 0.05). Data are expressed as the mean ± s.d.
Discussion
T1DM increases the risk of neurological complications and lowers functioning in several cognitive domains14,19, probably by disrupting microvascular networks32. In this study, we demonstrate that T1DM in both male and female mice is associated with a 2–4-fold increase in stalled cortical capillaries relative to healthy controls. Of note, treating diabetic mice with insulin was not sufficient to fully normalize the risk of capillary obstructions, which is consistent with other studies showing that partial normalization of blood glucose levels does not necessarily rescue diabetes-related neuropathology33–35. Assuming that there are approximately 10,000–20,000 capillaries per mm3 (ref. 10), our experiments suggest that about 0.47–0.93% of capillaries in healthy mice or 1.82–3.64% in diabetic mice are stalled at any given time. These estimates closely match other in vivo studies in healthy mice, including those using awake imaging preparations, and slightly higher rates in diabetic mice relative to those found in mouse models of AD9,13,36. Furthermore, most stalls were evident in the first to fourth order branches off AVs9,13.
We fluorescently tagged leucocytes using a CD45.2 antibody, which labels 95% of leucocytes and does not cause any cell depletion24. Our experiments revealed that most stalls in diabetic mice consisted of erythrocytes rather than leucocytes, thereby arguing that adherent leucocytes were not the primary driver of stalled capillaries. This contrasts with studies in diabetic retina, where monocytes and neutrophils plugged capillaries37. However, as there was significant tissue damage in those studies, one cannot know whether leucocyte-plugged capillaries contributed to injury or were a result of it. The idea of a ‘sticky’ endothelium is well appreciated in the literature about diabetes38 and could explain why most stalls were occluded with erythrocytes. Indeed, there are reports in other diseases such as thrombocythaemia, where erythrocytes stalled most capillaries11. Therefore, while leucocytes and in particular neutrophils, have a major role in stalling in mouse models of stroke and AD12,13, their role may be secondary to endothelial dysfunction in diabetes.
Chronic inflammation is a contributing factor to capillary dysfunction. Consistent with previous studies, we showed that T1DM, especially if poorly controlled, is associated with elevated levels of inflammatory blood cytokines and chemokines39–41. In particular, IL-10 levels were persistently elevated as in previous reports in eye and blood in patients with T1D and animal models40,42–44. IL-10 levels are elevated in the blood of other conditions, such as lipopolysaccharide challenge, adverse reactions to CAR T cell therapy and coronavirus disease 2019 (refs. 45–47). One limitation with previous studies was the correlational nature of IL-10 changes, that is, it was unknown whether it was serving a protective or pathogenic role. Thus, we adopted orthogonal approaches to stimulate or inhibit IL-10 signalling. Contrary to initial expectations of a protective effect as shown with infection, stroke or endotoxin challenge20,21, IL-10 signalling had a pathogenic role in capillary obstruction in diabetic mice. Consistent with this, inhibiting IL-10 receptor signalling broadly with antibodies or endothelial-cell-specific knockdown lowered the density of obstructed capillaries. The notion that IL-10R signalling could have a pathogenic role in certain disease states is not without precedent given that stimulating IL-10 in autoimmune diseases, such as lupus or multiple sclerosis, can aggravate symptoms and sequelae27. Studies revealed that IL-10 can worsen cognitive decline in mouse models of AD48,49. Indeed IL-10 signalling is complex and there are scattered reports showing that it can promote inflammatory processes50, such as accelerating disease progression in NOD mice51. Thus, our findings reinforce the notion that IL-10 signalling can have both protective and deleterious roles depending on the specific cell, organ or disease.
To our knowledge, this is the first study to show that blocking IL-10 signalling can exert a protective effect on cerebral microcirculation and cognition, at least in diabetes. Our qPCR data and knockdown experiments also indicate that endothelial IL-10 receptors, which would be in direct contact with elevated IL-10 in blood serum, have an important role. The protective effects of inhibiting IL-10 signalling cannot be explained by a systemic change in blood glucose because levels were similar in all diabetic mice, regardless of treatment (Extended Data Fig. 4). While IL-10 receptors are also expressed in neutrophils and microglia, which have been implicated in regulating the microvasculature of the diabetic retina52,53, these cells probably were not major contributors given that knockdown of IL-10 receptors in neutrophils had no effect on capillary obstruction. Furthermore, our viral therapy (using AAV.BR1.iCre) was restricted to the endothelium and did not target microglia. However, as we did not manipulate IL-10 receptors in every possible cell type, we concede that IL-10 signalling in other cells (for example, monocytes) could be a factor.
How might treatments that target IL-10 signalling work? Capillary stalling is regulated through multiple mechanisms, including changes in blood flow or tone, adhesion proteins in endothelial cells and leucocytes, and the glycocalyx36. Our data show that treating diabetic mice with IL-10R antibodies improved CBF and dilated capillary branches by 25%. Thus, endothelial IL-10 signalling could modulate vascular tone through astrocytes54 or contractile mural cells55,56. Indeed, a 25% increase in diameter could greatly increase blood flow2 and thereby push out erythrocyte-plugged capillaries on the venous side. From a signalling perspective, addition of IL-10 to cell culture can suppress vasoactive messengers, such as nitric oxide (NO), while IL-10 neutralizing antibodies can increase NO production57,58. IL-10 signalling could also augment the expression of cell adhesion or tight junction proteins, which are upregulated and downregulated, respectively, in mice with elevated rates of capillary stalling36. Our transcriptomic data indicated that inhibiting IL-10 reduced endothelial expression of focal adhesion and platelet activation genes (for example, vinculin, Fyn, collagen 4, ParvB). Other work showed that stimulating endothelial cells with IL-10 enhances expression of vascular cell adhesion molecule 1, especially in the presence of activated leucocytes59. IL-10 also increases BBB permeability by lowering endothelial resistance and occludin expression60. Thus, blocking IL-10 signalling could alleviate stalling through multiple avenues that future studies could explore.
Alleviating capillary stalling with neutrophil or vascular endothelial growth factor-targeting antibodies can rescue cognitive function in mice modelling AD13,36. In this study, we searched for alternate targets for diabetic mice because most stalled capillaries were not plugged with leucocytes. Therefore, we used the most translatable approach of treating with an IL-10R neutralizing antibody. This treatment led to a 63% reduction in stalling and improved learning, memory and cognitive flexibility. One could then ask, how does unblocking a relatively small fraction of capillaries affect cognition? Previous data suggested that stalling 2–4% of capillaries can lead to a 5–20% decrease in blood flow and improved cognition in AD mice13,61. Our microsphere experiments show that diabetes also increased the fraction of long-lived (~3-day) capillary obstructions. Previously, we showed that long-lived obstructions augment blood flow and diameter in connected capillaries for up to 2 weeks, and often resulted in vessel pruning10. Furthermore, capillary occlusion and subsequent rarefaction can lead to progressive degeneration of apical dendrites in cortical neurons62 or a reduction in local neural activity. Thus, it is reasonable to believe that greatly elevated numbers of short-lasting or long-lasting capillary obstructions in diabetic mice could impact cognitive function and thereby represent a treatable target for ameliorating cognitive impairment.
There are caveats and limitations to our study. First, blood glucose levels in diabetic mice were not completely normal when treated with insulin. Therefore, it is possible that complete control could have mitigated some of the microcirculatory problems. Second, although we used microspheres as a high-throughput proxy for assessing microcirculatory disturbances and then confirmed many of these findings in vivo, microspheres lack the deformability and cell signalling that would be present in blood cells. Thus, microsphere data should be interpreted with some caution and may not be suitable for understanding how cell adhesion proteins mediate stalling in disease states. Lastly, given that IL-10 has pleiotropic effects and that a recent study showed that ablating IL-10 can improve insulin sensitivity in adipose tissue63, future studies are needed to determine whether the benefits of IL-10 inhibition could be mediated through changes in insulin signalling.
Methods
Animals
Adult male and female mice (2–6 months old) on C57BL/6J (cat. no. 003658, The Jackson Laboratory) background were used. For AAV-mediated knockdown of IL-10 receptors in endothelial cells, we used Il10raloxP/loxP mice (cat. no. 028146, The Jackson Laboratory) where loxP sites are expressed on exon 3 of the Il10ra gene. To manipulate IL-10 signalling in neutrophils, we crossed Il10raloxP/loxP mice with the constitutive Cre driver mouse line Mrp8/S100a8-Cre (cat. no. 021614, The Jackson Laboratory). Previous work showed that the Mrp8/S100a8-Cre line has high specificity for neutrophils64,65. All mice were housed in groups on a 12-h light–dark cycle in ventilated racks in a humidity-controlled (40–55%) and temperature-controlled room (21–23 °C). Mice were provided food (Picolab Rodent diet 20, cat. no. 50553) and water ad libitum. All experiments complied with the guidelines set by the Canadian Council on Animal Care and were approved by the local university Animal Care Committee. Reporting of this work complies with Animal Research: Reporting of In Vivo Experiment guidelines.
Mouse models of T1D and insulin treatment
T1D was induced using two low-dose intraperitoneal injections of STZ (75 mg kg−1 per injection, cat. no. S0130, Sigma-Aldrich) dissolved in 50 mM (pH 4.5) sodium citrate buffer over consecutive days. Mice that had received STZ injections but did not exceed the hyperglycaemic threshold (>15 mM) were given a single, additional dose of STZ. Non-diabetic controls were administered sodium citrate buffer alone. Mice were given 5% sucrose water overnight after each day’s injection to prevent sudden hypoglycaemia. Blood glucose levels were measured (Accu-Chek Aviva, Roche Diabetes Care) every week by fasting mice for 2–3 h and then withdrawing a drop of blood from the tail. A subset of diabetic mice had one slow-release insulin pellet (0.1 U 24 h−1 per implant, LinBit) implanted subcutaneously under anaesthesia between the scapulae 1 week after confirmation of hyperglycaemia. Additional insulin pellets were implanted if glucose levels were greater than 15 mM. To examine blood cytokine expression in a genetic model of T1D, we used ten NOD mice (eight female and two male, cat. no. 001976, The Jackson Laboratory) and 13 non-diabetic control mice (seven female and six male, cat. no. 002050, The Jackson Laboratory). Mice were housed for 4.5–6 months, until they spontaneously developed hyperglycaemia.
Cranial window surgery
Mice were anaesthetized with isoflurane (2% for induction and 1.3% for maintenance) in medical air at a flow rate of 0.7 l min−1. Body temperature was maintained at 37 °C throughout the procedure. After subcutaneous injection of lidocaine, the scalp was cut and retracted. A custom-made metal ring to head fix mice during imaging (~1 g in weight, outer diameter 11.3 mm, inner diameter 7.0 mm, height 1.5 mm) was positioned over the right somatosensory cortex and secured to the skull with Metabond adhesive. A circular area (diameter of ~4–5 mm) of skull was thinned using a high-speed dental drill; ice-cold HEPES-buffered artificial cerebrospinal fluid was applied periodically for cooling. Fine forceps removed the skull and a 5 or 6 mm circular coverslip was placed over the exposed brain and secured using cyanoacrylate adhesive. After the procedure, mice were injected intraperitoneally with 0.03 ml of 2% dexamethasone to reduce acute inflammation.
Imaging and analysis of cortical microcirculation
To minimize surgery-induced inflammation66, in vivo two-photon imaging began ~6 weeks after the cranial window surgeries and 2–4 weeks after diabetes induction (Fig. 1a). Mice were lightly anaesthetized with isoflurane (1% for imaging), with the metal ring clamped into a custom frame and body temperature maintained at 37 °C. Fluorescein or Texas Red-labelled dextran (0.1 ml of 1–5% of 70 kDa dextran solution in saline; cat. no. 46945, Sigma-Aldrich, or cat. no. D1830, Thermo Fisher Scientific) was injected intravenously to visualize blood flow. To image leucocytes24, a subset of mice were injected with 0.1 ml solution containing 0.4 mg kg−1 FITC anti-mouse CD45.2 antibody (clone 104, cat. no. 109806, BioLegend) and 1% Texas Red solution. Neutrophils were labelled with an injection of 0.2 mg kg−1 Alexa Fluor 647 anti-mouse Ly-6G (clone 1A8, cat. no. 127609, BioLegend).
High-resolution two-photon image stacks of flowing and stalled capillaries were generated using an Olympus FV1000MPE multiphoton laser scanning microscope equipped with a mode-locked Ti:sapphire femtosecond laser (Mai Tai XF Deep See, Spectra-Physics). The laser was tuned to 800 or 850 nm for the excitation of FITC or Texas Red dextran, respectively, or 940 nm for tandem imaging of FITC–CD45.2-labelled leucocytes and Texas Red dextran-labelled blood plasma. Excitation power measured at the back aperture of the objective was typically between 17 and 60 mW, and was adjusted to achieve similar levels of fluorescence across imaging sessions. Images were acquired either with a ×40 Olympus IR-LUMPlanFl water-immersion objective (numerical aperture (NA) = 0.8) or a ×20 Olympus water-immersion objective (NA = 0.95), using the Olympus FV10-ASW software. Three to four imaging areas per mouse were chosen based on proximity to the forelimb or hindlimb area of the somatosensory cortex. During each imaging session, blood vessels were imaged at a frame rate of 3.25 s per frame, to a depth of 200–300 μm below the pial surface. Image stacks were collected with the following parameters: (1) ×40 objective: 1.5-μm Z-steps covering an area of 317.4 × 317.4 μm (0.31 μm per pixel); or (2) ×20 objective: 1.25-μm Z-steps covering an area of 489.5 × 489.5 μm (0.48 μm per pixel). After imaging, mice were monitored while they recovered under supplementary heat67.
Capillary stalls were identified by observers blinded to condition by first examining maximal Z-projection images (20–25 images) of cortical vessels. Capillaries that did not show any evidence of blood plasma streaks (streaks caused by cells moving through the lumen), and those that possessed dark gaps in the lumen (putative unlabelled RBCs) or FITC-labelled leucocytes, were considered candidate stalls. These stalls were then followed up and verified using manual scrolling through three-dimensional image stacks. A stalled capillary was determined if there was an absence of RBC streaks or presence of static cells (RBCs or leucocytes) plugging the capillary for at least three imaging frames (minimum stall duration of 6.5 s), similar to previous criteria13. To determine the branch order of stalled capillaries, vessels were traced back to the nearest PA or venule, with the aid of brightfield images of the cortical surface.
RBC flux through a given capillary was estimated from raster-scanned two-photon imaging stacks collected with a ×20 objective: 1.25-μm Z-steps covering an area of 489.5 × 489.5 μm (0.48 μm per pixel). Flux measurements were restricted to capillaries with a diameter of 4 µm or less oriented vertically to the imaging plane (along the y-scanning axis) and mostly in one or two Z-planes, with little tortuosity. This was done to ensure sufficiently uniform and consistent time intervals to detect RBCs (black pixels followed by bright-plasma-related pixels) running down the middle of the vessel lumen. For a given vessel, two frames were projected and a two-pixel-wide linear profile was manually traced down the centre of the vessel in ImageJ. The plot profile of this line was then saved as a .csv file, which was imported into a Python 3 Jupyter Notebook (code available at https://github.com/preeson/Sharma-et-al-Nat-Met-2024-Flux-Estimation). These ‘pseudo-linescans’ were first inverted (so unlabelled RBC streaks were peaks) and smoothed using Scipy Savitzky–Golay filtering (window length of five, poly order of two), mean-subtracted and normalized. The number of RBCs was then estimated by applying the Scipy find_peaks algorithm, with a minimum height of zero, minimum prominence of 0.005 and minimum distance between peaks of three pixels (https://docs.scipy.org/doc/scipy/reference/generated/scipy.signal.find_peaks.html). The number of RBCs in the pseudo-linescan was defined as the number of detected peaks; the time interval was defined as the number of pixels in the line times a single line raster scan speed of 3.17 ms per line. The flux was then calculated as the number of RBCs per second. Our analysis yielded average RBC fluxes that were in the ranges reported by others68. For each animal, six or more vessels matching our criteria were selected for each time point, maintaining the same vessels across time as much as possible.
Microsphere assay of capillary obstruction
As described previously5,10, we injected fluorescent microspheres into mice to examine experimental differences in capillary obstruction susceptibility. Mice were briefly anaesthetized with 1.5% isoflurane and injected intravenously with 3 µl g−1 body weight fluorescent microspheres with a 5-μm diameter (1% solids; cat. no. FCDG008, Bangs Laboratories). Importantly, experimental groups were run in parallel and injected from the same stock solution of microspheres to minimize cohort-to-cohort variability. Mice were monitored and recovered under supplementary heat. We did not observe any abnormal behaviour or morbidity after the injections. Three days after the injection, mice were deeply anaesthetized and the brain was extracted (without perfusion) and immersed in 4% paraformaldehyde overnight before being transferred to 0.1 M PBS. Brains were sectioned in the coronal plane on a Leica vibratome (T1000) at 50-µm thickness. Every sixth section was mounted onto a slide and cover-slipped with Fluoromount-G. Fluorescent microspheres were imaged on an upright Olympus BX51 microscope with a ×2 Olympus Plan objective (NA = 0.05) using green fluorescent protein (GFP) excitation and emission filter sets on an Olympus DP73 digital charge-coupled device camera using the CellSens software. Images were taken from +2.7 to −3.5 mm from bregma, thereby covering much of the mouse forebrain. Using Image J (v.1.53q), regions of interest were manually drawn over 13 different brain regions, as described previously5. Automated counting of microspheres across the mouse forebrain or in each region was achieved by thresholding pixels of 67% maximum intensity. The density of capillary obstructions was expressed as the number of obstructions per mm3.
Manipulation of IL-10 signalling in vivo
To broadly inhibit IL-10 or IL-10 receptor signalling in vivo, we randomly assigned diabetic mice to receive an intravenous injection of 250 µg per mouse of IL-10 neutralizing antibody (cat. no. BE0049, Bio X Cell), IL-10R neutralizing antibody (InVivoMAb anti-mouse IL-10R, CD210, clone 1B1.3A, cat. no. BE0050, Bio X Cell) or equivalent volume of isotype control antibody (cat. no. BE0088, Bio X Cell). Given the durable activity of neutralizing antibodies, injections were always spaced out by 3–4 days (that is, two injections per week). For the IL-10 stimulation experiments, non-diabetic mice were injected intravenously with either recombinant mouse IL-10 protein (0.5 µg per mouse; cat. no. 417-ML-005/CF, R&D Systems) or albumin (0.5 µg as control) dissolved in saline over two consecutive days before injection of fluorescent microspheres, as described above. To rule out the possibility that albumin (in control mice) could affect capillary plugging, an additional group was injected with saline (n = 4 mice) or 0.5 µg albumin mixed in saline (n = 5 mice). For endothelial cell knockdown of IL-10Ra receptors, Il10raloxP/loxP mice were injected intravenously with AAV-BR1-iCre or enhanced GFP control (20 µl per mouse at 5.0 × 1012 GC ml−1)28, 3 weeks before the fluorescence microsphere assay.
Behavioural testing
Behavioural tests were performed at 4 and 8 weeks after induction of diabetes, with each testing period taking 1 week to complete (Fig. 6a). The 4-week time point was used to evaluate the initial impact of diabetes on behaviour; the 8-week time point was used to determine whether IL-10R blocking antibody treatment reversed any behavioural impairments.
NOR task
Mice were first habituated to a Plexiglass box (30 × 30 × 30 cm) devoid of any objects for 5 min. On the day of testing, mice were allowed to explore two identical objects, 12 cm apart from each other, for 5 min. The box and objects were wiped with 70% v/v ethanol to remove odours after each trial. Six hours later, a novel object was substituted for one of the familiar objects and mice were left to explore the objects for 5 min. Using the EthoVision software (v.11.5.1020, 2015; Noldus Information Technology), the amount of time mice spent exploring with the snout pointed in each object zone was determined as a percentage (time spent exploring the novel object divided by the total amount of time spent exploring objects). Similarly, the preference ratio for the frequency of visits to the novel zone was calculated.
Morris water maze
A hidden platform was placed 1 cm below the surface in a water-filled circular pool (100-cm diameter, ~22 °C temperature). Non-toxic white paint was added to the water to conceal the platform. Mice were trained to locate the hidden platform by swimming using distinct visual cues placed around the maze. These cues varied in shape and were kept constant throughout. The EthoVision software was used to score escape latency (s) and swim speeds (cm s−1) to the platform. The trial began with the mouse being placed in one quadrant facing the pool wall. The trial ended once the mouse located and stood on the platform for 3 s, or after a maximum of 2 min had elapsed without finding the platform. If the latter occurred, the experimenter placed the mouse on the platform and allowed it to remain there for 5 s. Each training session consisted of four trials, with each trial having a different starting point (south-east, north-west, north-east and south-west). Two training sessions were performed on each testing day, for a total of four training sessions over the two days. As the water maze was conducted twice in the same animals, the hidden platform was located in different quadrants for the 4-week and 8-week testing sessions. Two days after the mice completed the initial platform learning trials, the hidden platform was removed and mice were left to explore for 2 min to assess reference memory (that is, the probe test). The amount of time spent in each quadrant was recorded and a preference ratio was calculated (time spent in the target quadrant divided by the time spent in the other three quadrants). Three days after the probe test, we tested cognitive flexibility by switching the location of the hidden platform to a new location, which we refer to as the ‘platform reversal learning test’. Training sessions were run in the same manner as described above for initial platform learning.
Visible platform test
At 8 weeks, a platform that visually contrasted with the water was raised above the water line and the latency (s) to find the platform was recorded.
Tape removal test
For each session of testing, mice underwent three trials that involved placing circular pieces of tape (5-mm diameter) onto the palmar surface of both forepaws. Each mouse was filmed from underneath the glass cylinder and the latency to remove each piece of tape was recorded and averaged67.
Open field test
Mice were placed in a 100-cm diameter arena and the total distance travelled (cm) was recorded and tracked using the EthoVision software. A 5-min trial was conducted for each mouse at both 4 and 8 weeks.
Elevated plus maze
Mice were placed in a raised plus-shaped platform, with two arms having high borders (closed arms) and two without (open arms). Each mouse was run through one trial for 5 min. The EthoVision software was used to track the mouse’s movements; the percentage of time spent in the open arm was calculated.
Laser Doppler measurements of CBF
A laser Doppler was used to assess regional CBF in response to vasoactive stimuli69. Under urethane anaesthesia (1.25 g kg−1), the skull was thinned over the right hemisphere. During the experiment, oxygen saturation and heart rate were assessed in a subset of mice (n = 6–7 mice per group) by placing a mouse pulse oximetry sensor around the thigh (STARR Life Sciences). Measurements were collected at 15 Hz and averaged over a 5.5-min period. The laser Doppler probe (MoorVMS-LDF1, PC v.2.2, Moor Instruments) was placed approximately 1 mm above the skull with a stereotaxic arm. Three different stimuli were used: (1) inhalation of 5% CO2 mixed in medical air for 60 s (×1 trial); (2) vibrotactile hindlimb sensory stimulation (100 Hz stimulation for 10 s, ×6 trials); (3) 30-s inhalation of 1% isoflurane (mixed in medical air, ×1 trial). For each trial, we obtained a stable baseline before presenting each stimulus and recorded changes in blood flow. Using the pCLAMP software, components of the CBF response, such as peak amplitude, latency to peak and a.u.c., were measured from 0 to 120 s after CO2 exposure, 0–15 s after sensory stimulation and 0–180 s after isoflurane.
Measurements of immune cytokines and chemokines
Mice were deeply anaesthetized; blood was collected and allowed to clot at room temperature for 10 min and then spun at 1,500 rpm for 10 min at 4 °C. The supernatant was collected, flash-frozen in liquid nitrogen and stored at −80 °C. Blood serum samples from control and diabetic mice (at 8 weeks) were thawed and assayed using the Meso Scale Discovery U- PLEX Biomarker Group 1 (mouse) 29-Plex panel assay (K15355K-1; lot no. 393914-393917) according to the manufacturer’s instructions to measure serum cytokine concentrations. Samples were run in duplicate; plates were read using the QuickPlex SQ 120MM instrument and data were analysed using the Discovery Workbench v.4.0 software (Meso Scale Discovery). The lower limits of calibrator detection for all assays were similar to the product datasheets, demonstrating the sensitivity and robustness of the U-PLEX method. For serum sample signals in the calibrator detection range, the average calculated sample concentration percentage coefficient of variation was 11%, showing the precision of the assays for quantifying changes in inflammatory response to diabetes. Selected serum cytokines (IL-1β, IL-6, IL-10, MCP-1 (CCL2), KC (CXCL1), IL-17 and MIG1 (CXCL9)) were also analysed using a cytometric bead array in healthy controls and diabetic mice at 4 weeks’ hyperglycaemia. Samples and standards were incubated with capture beads and detection reagent according to the manufacturer’s instructions (BD Biosciences). Samples were analysed with a CytoFLEX Flow Cytometer and the CytExpert software. Mean sample fluorescence values were interpolated to standard curves to derive cytokine concentration in pg ml−1.
Endothelial cell separation
Mice were deeply anaesthetized; the cortex and cerebellum from the left hemisphere were immediately dissected and collected in 2 ml RNase-free microfuge tubes (cat. no. AM12475, Thermo Fisher Scientific) containing 1 ml HBSS with no calcium and magnesium (cat. no. 14175095, Thermo Fisher Scientific). The mechanical dissociation of brain tissue was performed using the Adult Brain Dissociation Kit (cat. no. 130-107-677, Miltenyi Biotec) according to the manufacturer’s instructions. Briefly, the cortex and cerebellum were homogenized by gently pipetting up and down approximately ten times with a 1-ml pipette at 37 °C. The recovered homogeneous cell mixture was gently applied to a smart strainer (cat. no. 130-098-462, Miltenyi Biotec) to remove the connective tissue. Samples were always kept on ice unless otherwise indicated. Myelin was removed using the Myelin Removal Kit (cat. no. 130-096-733, Miltenyi Biotec) according to the manufacturer’s instructions and then passed through LS columns (cat. no. 130-042-401, Miltenyi Biotec) using the Quadro-MACS separator (cat. no. 130-090-976, Miltenyi Biotec). After three washes with Auto-MACS Rinsing Solution (cat. no. 130-091-222, Miltenyi Biotec) containing 0.5% BSA (cat. no. 130-091-376, Miltenyi Biotech), the flow-through was centrifuged for 10 min and resuspended in Auto-MACS Rinsing Solution containing 0.5% BSA. Microglia were removed from the total cell suspension using CD45 microbeads (cat. no. 130-052-301, Miltenyi Biotech) by passing the cell mixture through LS columns. The flow-through containing CD45− cells was then centrifuged for 10 min. The cell pellet was resuspended in Auto-MACS Rinsing Solution with 0.5% BSA and incubated with CD31 microbeads (cat. no. 130-097-418, Miltenyi Biotech) and passed through MS column (cat. no. 130-042-201, Miltenyi Biotech) using the Octo-MACS separator (cat. no. 130-042-108, Miltenyi Biotec). After three washes with Auto-MACS Rinsing Solution with 0.5% BSA, column-bound CD31+ endothelial cells were collected by adding 1 ml Auto-MACS Rinsing Solution with 0.5% BSA. The collected sample was centrifuged at maximum speed for 10 min. After discarding the supernatant, the endothelial cell pellet was flash-frozen and kept at −80 °C.
RNA extraction and analysis
Total RNA was extracted using the RNeasy Mini Kit (cat. no. 74104, QIAGEN) with a deoxyribonuclease (cat. no. 79254, QIAGEN) treatment step according to the manufacturer’s instructions either from flash-frozen brain tissue or isolated cells. RNA purity and concentrations were estimated using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific) and samples were stored at −80 °C. Complementary DNA (cDNA) was prepared using the High-Capacity cDNA Synthesis Kit (cat. no. 4368814, Applied Biosystems) from 50 ng RNA with the help of the Bio-Rad T100 thermocycler (Bio-Rad Laboratories). cDNA was diluted fivefold for qPCR with reverse transcription (RT–qPCR) analysis. Primer specificities were calculated both online using the NCBI primer blast and experimentally using melt curve analysis. A five-point tenfold serial dilution was used to test primer efficiency; those that achieved efficiencies between 90% and 110% were used for gene expression analysis. RT–qPCR was carried out using a 10-μl reaction mixture containing 1 μl cDNA, 0.5 μl of each primer (10 μm stock), 3 μl RNase, DNase-free water and 5 μl of SYBR Green Master Mix (Applied Biosystems). The thermocycling conditions used were 50 °C (2 min), 95 °C (2 min), followed by 40 cycles of 95 °C denaturation (15 s) and 60 °C/62 °C annealing (1 min). Fluorescence signals were acquired using the StepOne plus system and data were analysed using the Design and Analysis Software v.2.4.3 (Applied Biosystems). Triplicate reactions were performed for each sample and Ct values were averaged and normalized to the geometric mean of TATA-binding protein and hypoxanthine phosphoribosyltransferase 1 using the comparative method to calculate relative mRNA levels. The forward and reverse primers used for RT–qPCR were as follows: Tbp (housekeeping gene): CCCCACAACTCTTCCATTCT and GCAGGAGTGATAGGGGTCAT; Hprt (housekeeping gene): AGCCTAAGATGAGCGCAAGT and TTACTAGGCAGATGGCCACA; Il10ra (exon 3-specific): AACCTGGAATGACATCCATATC and CCACTGTGAAGCGAGTCTCAGT; Vegfr2: TTTGGCAAATACAACCCTTCAGA and GCAGAAGATACTGTCACCACC; Tie2: GAGTCAGCTTGCTCCTTTATGG and AGACACAAGAGGTAGGGAATTGA; Cd31: ACGCTGGTGCTCTATGCAAG and TCAGTTGCTGCCCATTCATCA; Cdh5: CCACTGCTTTGGGAGCCTT and GGCAGGTAGCATGTTGGGG; Tmem119: CTTCACCCAGAGCTGGTTCCATA and CCGGGAGTGACACAGAGTAG; Cd45: GTTTTCGCTACATGACTGCACA and AGGTTGTCCAACTGACATCTTTC; NeuN: ATCGTAGAGGGACGGAAAATTGA and GTTCCCAGGCTTCTTATTGGTC.
RNA sequencing and bioinformatics
RNA sequencing (RNA-seq) was performed by Novogene according to their standardized procedures. For library construction, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. After fragmentation, first-strand cDNA was synthesized using random hexamer primers, followed by second-strand cDNA synthesis using either deoxyuridine triphosphate for adirectional library or deoxythymidine triphosphate for a non-directional library. For the non-directional library, samples were ready after end repair, A-tailing, adaptor ligation, size selection, amplification and purification. For the directional library, samples were ready after end repair, A-tailing, adaptor ligation, size selection, USER enzyme digestion, amplification and purification. The library was checked with Qubit and real-time PCR for quantification, and bioanalyzer for size distribution detection. Quantified libraries were pooled and sequenced on Illumina platforms, according to the effective library concentration and data amount.
For data quality control, raw data (raw reads) of .fastq format were first processed through in-house Perl scripts. In this step, clean data (clean reads) were obtained by removing reads containing adaptor, reads containing poly-N and low-quality reads from raw data. At the same time, Q20, Q30 and GC content were calculated using the clean data. All downstream analyses were based on high-quality clean data. The reference genome and gene model annotation files were downloaded from the genome website directly. The index of the reference genome was built using HISAT2 v.2.0.5; paired-end clean 1 reads were aligned to the reference genome using HISAT2 v.2.0.5. We selected HISAT2 as the mapping tool because HISAT2 can generate a database of splice junctions based on the gene model annotation file and thus a better mapping result than other non-splice mapping tools70.
Quantification of gene expression was done using featureCounts v.1.5.0-p3 to count the read numbers mapped to each gene71. The expected number of fragments per kilobase of transcript sequence per millions base pairs of each gene was calculated based on the length of the gene and the read counts mapped to a particular gene. The fragments per kilobase of transcript sequence per millions base pairs consider the effect of sequencing depth and the gene length for the read count at the same time; it is currently the most commonly used method for estimating gene expression levels. Differential expression analysis of the two groups was performed using the DESeq2 package (v.1.20.0). DESeq2 provides statistical routines to determine differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting P values were adjusted using the Benjamini–Hochberg approach for controlling the false discovery rate. Genes with Padj ≤ 0.05 found using DESeq2 were assigned as differentially expressed. A corrected P ≤ 0.05 and a log2 fold change of 1.3 were set as the threshold for significant differential expression. Gene Ontology enrichment analysis of DEGs was implemented using the cluster Profiler R package, in which gene length bias was corrected. Gene Ontology terms with a corrected P ≤ 0.05 were considered significantly enriched by DEGs. We used the cluster Profiler R package to test the statistical enrichment of DEGs in KEGG pathways.
Statistics
All data were analysed using Prism v.7 and v.8 (GraphPad Software) (Research Resource Identifier: SCR_002798). Data distribution was assumed to be normal but this was not formally tested. Student’s t-tests (paired or unpaired, as appropriate) were used to analyse simple between-group differences (that is, when only two or three groups are compared on a single factor). Two-way ANOVAs were used to compare differences between treatment groups across time, testing session or brain region. Significant main effects from the ANOVAs were analysed with a Tukey’s multiple-comparisons test. Statistical outliers were identified and excluded based on a ROUT or Grubbs test. P < 0.05 was considered statistically significant. No statistical methods were used to predetermine sample sizes but our sample sizes are similar to those reported in previous publications5,10,35,67. All experimental values are presented as the mean ± s.e.m. unless otherwise indicated.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Differential endothelial gene expression between diabetic mice treated with IL-10R antibody versus isotype antibody controls.
Differential endothelial gene expression between diabetic mice treated with isotype antibody versus non-diabetic controls.
Source data
Statistical source data for Figs. 1–8 and Extended Data Figs. 1–7.
Acknowledgements
We thank K. Delaney for his input, and A. Hentze and T. Yang for managing the mouse colony. We also thank D. Attwell (UCL) for thoughtful discussions regarding the work. This research was supported by operating, salary and equipment grants to C.E.B. from the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada and the Natural Sciences and Engineering Research Council. S.S. and P.L.R. were supported by a postdoctoral fellowship from the Michael Smith Foundation for Health Research.
Extended data
Author contributions
C.E.B. and S.S. conceived the study and wrote the manuscript. S.S., M.C., P.L.R., K.N., R.B., A.P.C., T.P.B., R.D.F., L.A.R., J.K. and C.E.B. provided key intellectual insights and reagents, performed the experiments or analysed the data.
Peer review
Peer review information
Nature Metabolism thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Ashley Castellanos-Jankiewicz, in collaboration with the Nature Metabolism editorial team.
Data availability
Further information and data requests will be entertained upon reasonable request to the corresponding author C.E.B. (brownc@uvic.ca). Source data are provided with this paper.
Code availability
The custom-written code for the RBC flux estimation is available at https://github.com/preeson/Sharma-et-al-Nat-Met-2024-Flux-Estimation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s42255-024-01159-9.
Supplementary information
The online version contains supplementary material available at 10.1038/s42255-024-01159-9.
References
- 1.Attwell, D. & Laughlin, S. B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab.21, 1133–1145 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Davis, H. & Attwell, D. A tight squeeze: how do we make sense of small changes in microvascular diameter? J. Physiol.601, 2263–2272 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harraz, O. F., Longden, T. A., Dabertrand, F., Hill-Eubanks, D. & Nelson, M. T. Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP2 depletion. Proc. Natl Acad. Sci. USA115, E3569–E3577 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zambach, S. A. et al. Precapillary sphincters and pericytes at first-order capillaries as key regulators for brain capillary perfusion. Proc. Natl Acad. Sci. USA118, e2023749118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schager, B. & Brown, C. E. Susceptibility to capillary plugging can predict brain region specific vessel loss with aging. J. Cereb. Blood Flow Metab.40, 2475–2490 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blinder, P. et al. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci.16, 889–897 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji, X. et al. Brain microvasculature has a common topology with local differences in geometry that match metabolic load. Neuron109, 1168–1187 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith, A. F. et al. Brain capillary networks across species: a few simple organizational requirements are sufficient to reproduce both structure and function. Front. Physiol.10, 233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdener, Ş. E. et al. Spatio-temporal dynamics of cerebral capillary segments with stalling red blood cells. J. Cereb. Blood Flow Metab.39, 886–900 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeson, P., Choi, K. & Brown, C. E. VEGF signaling regulates the fate of obstructed capillaries in mouse cortex. eLife7, e33670 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santisakultarm, T. P. et al. Stalled cerebral capillary blood flow in mouse models of essential thrombocythemia and polycythemia vera revealed by in vivo two-photon imaging. J. Thromb. Haemost.12, 2120–2130 (2014). [DOI] [PubMed] [Google Scholar]
- 12.El Amki, M. et al. Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep.33, 108260 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Cruz Hernández, J. C. et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat. Neurosci.22, 413–420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biessels, G. J., Deary, I. J. & Ryan, C. M. Cognition and diabetes: a lifespan perspective. Lancet Neurol.7, 184–190 (2008). [DOI] [PubMed] [Google Scholar]
- 15.McCrimmon, R. J., Ryan, C. M. & Frier, B. M. Diabetes and cognitive dysfunction. Lancet379, 2291–2299 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann, P. A., Knot, H. J., Stevenson, A. S. & Nelson, M. T. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ. Res.81, 996–1004 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Wang, S. et al. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. Geroscience42, 1387–1410 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandran, R. et al. Diabetic rats are more susceptible to cognitive decline in a model of microemboli-mediated vascular contributions to cognitive impairment and dementia. Brain Res.1749, 147132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma, S. & Brown, C. E. Microvascular basis of cognitive impairment in type 1 diabetes. Pharmacol. Ther.229, 107929 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med.15, 192–199 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Hickey, M. J., Issekutz, A. C., Reinhardt, P. H., Fedorak, R. N. & Kubes, P. Endogenous interleukin-10 regulates hemodynamic parameters, leukocyte-endothelial cell interactions, and microvascular permeability during endotoxemia. Circ. Res.83, 1124–1131 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Kinzenbaw, D. A., Chu, Y., Peña Silva, R. A., Didion, S. P. & Faraci, F. M. Interleukin-10 protects against aging-induced endothelial dysfunction. Physiol. Rep.1, e00149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hink, U., Tsilimingas, N., Wendt, M. & Münzel, T. Mechanisms underlying endothelial dysfunction in diabetes mellitus: therapeutic implications. Treat. Endocrinol.2, 293–304 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Faulhaber, L. D., D’Costa, O., Shih, A. Y. & Gust, J. Antibody-based in vivo leukocyte label for two-photon brain imaging in mice. Neurophotonics9, 031917 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silasi, G., She, J., Boyd, J. D., Xue, S. & Murphy, T. H. A mouse model of small-vessel disease that produces brain-wide-identified microocclusions and regionally selective neuronal injury. J. Cereb. Blood Flow Metab.35, 734–738 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaked, I. et al. A lone spike in blood glucose can enhance the thrombo-inflammatory response in cortical venules. J. Cereb. Blood Flow Metab.44, 252–271 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena, A. et al. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine74, 27–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Körbelin, J. et al. A brain microvasculature endothelial cell‐specific viral vector with the potential to treat neurovascular and neurological diseases. EMBO Mol. Med.8, 609–625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Østergaard, L. et al. Capillary transit time heterogeneity and flow-metabolism coupling after traumatic brain injury. J. Cereb. Blood Flow Metab.34, 1585–1598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iadecola, C. et al. The neurovasculome: key roles in brain health and cognitive impairment: a scientific statement from the American Heart Association/American Stroke Association. Stroke54, e251–e271 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munji, R. N. et al. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood–brain barrier dysfunction module. Nat. Neurosci.22, 1892–1902 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coucha, M., Abdelsaid, M., Ward, R., Abdul, Y. & Ergul, A. Impact of metabolic diseases on cerebral circulation: structural and functional consequences. Compr. Physiol.8, 773–799 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy, J. M. & Zochodne, D. W. Experimental diabetic neuropathy with spontaneous recovery: is there irreparable damage? Diabetes54, 830–837 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Biessels, G. J. et al. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res.800, 125–135 (1998). [DOI] [PubMed] [Google Scholar]
- 35.Mehina, E. M. F. et al. Invasion of phagocytic galectin 3 expressing macrophages in the diabetic brain disrupts vascular repair. Sci. Adv.7, eabg2712 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali, M. et al. VEGF signalling causes stalls in brain capillaries and reduces cerebral blood flow in Alzheimer’s mice. Brain145, 1449–1463 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schröder, S., Palinski, W. & Schmid-Schönbein, G. W. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am. J. Pathol.139, 81–100 (1991). [PMC free article] [PubMed] [Google Scholar]
- 38.Vinik, A. & Flemmer, M. Diabetes and macrovascular disease. J. Diabetes Complications16, 235–245 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Assar, M. E., Angulo, J. & Rodríguez-Mañas, L. Diabetes and ageing-induced vascular inflammation. J. Physiol.594, 2125–2146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beli, E. et al. CX3CR1 deficiency accelerates the development of retinopathy in a rodent model of type 1 diabetes. J. Mol. Med.94, 1255–1265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor, S. et al. Suppressing interferon-γ stimulates microglial responses and repair of microbleeds in the diabetic brain. J. Neurosci.38, 8707–8722 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cvitkovic, K. et al. Concentrations of selected cytokines and vascular endothelial growth factor in aqueous humor and serum of diabetic patients. Semin. Ophthalmol.35, 126–133 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Myśliwska, J. et al. High levels of circulating interleukin-10 in diabetic nephropathy patients. Eur. Cytokine Netw.16, 117–122 (2005). [PubMed] [Google Scholar]
- 44.Gouda, W., Mageed, L., Abd El Dayem, S. M., Ashour, E. & Afify, M. Evaluation of pro-inflammatory and anti-inflammatory cytokines in type 1 diabetes mellitus. Bull. Natl Res. Cent.42, 14 (2018). [Google Scholar]
- 45.Faulhaber, L. D. et al. Brain capillary obstruction during neurotoxicity in a mouse model of anti-CD19 chimeric antigen receptor T-cell therapy. Brain Commun.4, fcab309 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fruekilde, S. K. et al. Disturbed microcirculation and hyperaemic response in a murine model of systemic inflammation. J. Cereb. Blood Flow Metab.42, 2303–2317 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Messing, M. et al. Prognostic peripheral blood biomarkers at ICU admission predict COVID-19 clinical outcomes. Front. Immunol.13, 1010216 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakrabarty, P. et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron85, 519–533 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guillot-Sestier, M.-V. et al. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron85, 534–548 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lobo-Silva, D., Carriche, G. M., Castro, A. G., Roque, S. & Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflammation13, 297 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wogensen, L., Huang, X. & Sarvetnick, N. Leukocyte extravasation into the pancreatic tissue in transgenic mice expressing interleukin 10 in the islets of Langerhans. J. Exp. Med.178, 175–185 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Binet, F. et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science369, eaay5356 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Mills, S. A. et al. Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic retinopathy. Proc. Natl Acad. Sci. USA118, e2112561118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gordon, G. R. J., Mulligan, S. J. & MacVicar, B. A. Astrocyte control of the cerebrovasculature. Glia55, 1214–1221 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Hall, C. N. et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature508, 55–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartmann, D. A. et al. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci.24, 633–645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nemoto, Y. et al. Differential effects of interleukin-4 and interleukin-10 on nitric oxide production by murine macrophages. Inflamm. Res.48, 643–650 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Seyler, I., Appel, M., Devissaguet, J. P., Legrand, P. & Barratt, G. Modulation of nitric oxide production in RAW 264.7 cells by transforming growth factor-beta and interleukin-10: differential effects on free and encapsulated immunomodulator. J. Leukoc. Biol.62, 374–380 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Fiehn, C., Paleolog, E. M. & Feldmann, M. Selective enhancement of endothelial cell VCAM-1 expression by interleukin-10 in the presence of activated leucocytes. Immunology91, 565–571 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barabási, B. et al. Role of interleukin-6 and interleukin-10 in morphological and functional changes of the blood–brain barrier in hypertriglyceridemia. Fluids Barriers CNS20, 15 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue, Y. et al. Quantification of hypoxic regions distant from occlusions in cerebral penetrating arteriole trees. PLoS Comput. Biol.18, e1010166 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu, L. et al. Single-microvessel occlusion produces lamina-specific microvascular flow vasodynamics and signs of neurodegenerative change. Cell Rep.42, 112469 (2023). [DOI] [PubMed] [Google Scholar]
- 63.Rajbhandari, P. et al. IL-10 signaling remodels adipose chromatin architecture to limit thermogenesis and energy expenditure. Cell172, 218–233 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Passegué, E., Wagner, E. F. & Weissman, I. L. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell119, 431–443 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Abram, C. L., Roberge, G. L., Hu, Y. & Lowell, C. A. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods408, 89–100 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown, C. E., Boyd, J. D. & Murphy, T. H. Longitudinal in vivo imaging reveals balanced and branch-specific remodeling of mature cortical pyramidal dendritic arbors after stroke. J. Cereb. Blood Flow Metab.30, 783–791 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Motaharinia, M. et al. Longitudinal functional imaging of VIP interneurons reveals sup-population specific effects of stroke that are rescued with chemogenetic therapy. Nat. Commun.12, 6112 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee, J., Wu, W. & Boas, D. A. Early capillary flux homogenization in response to neural activation. J. Cereb. Blood Flow Metab.36, 375–380 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ouellette, J. et al. Vascular contributions to 16p11.2 deletion autism syndrome modeled in mice. Nat. Neurosci.23, 1090–1101 (2020). [DOI] [PubMed] [Google Scholar]
- 70.Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods5, 621–628 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Liao, Y., Smyth, G. K. & Shi, W. FeatureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics30, 923–930 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differential endothelial gene expression between diabetic mice treated with IL-10R antibody versus isotype antibody controls.
Differential endothelial gene expression between diabetic mice treated with isotype antibody versus non-diabetic controls.
Statistical source data for Figs. 1–8 and Extended Data Figs. 1–7.
Data Availability Statement
Further information and data requests will be entertained upon reasonable request to the corresponding author C.E.B. (brownc@uvic.ca). Source data are provided with this paper.
The custom-written code for the RBC flux estimation is available at https://github.com/preeson/Sharma-et-al-Nat-Met-2024-Flux-Estimation.