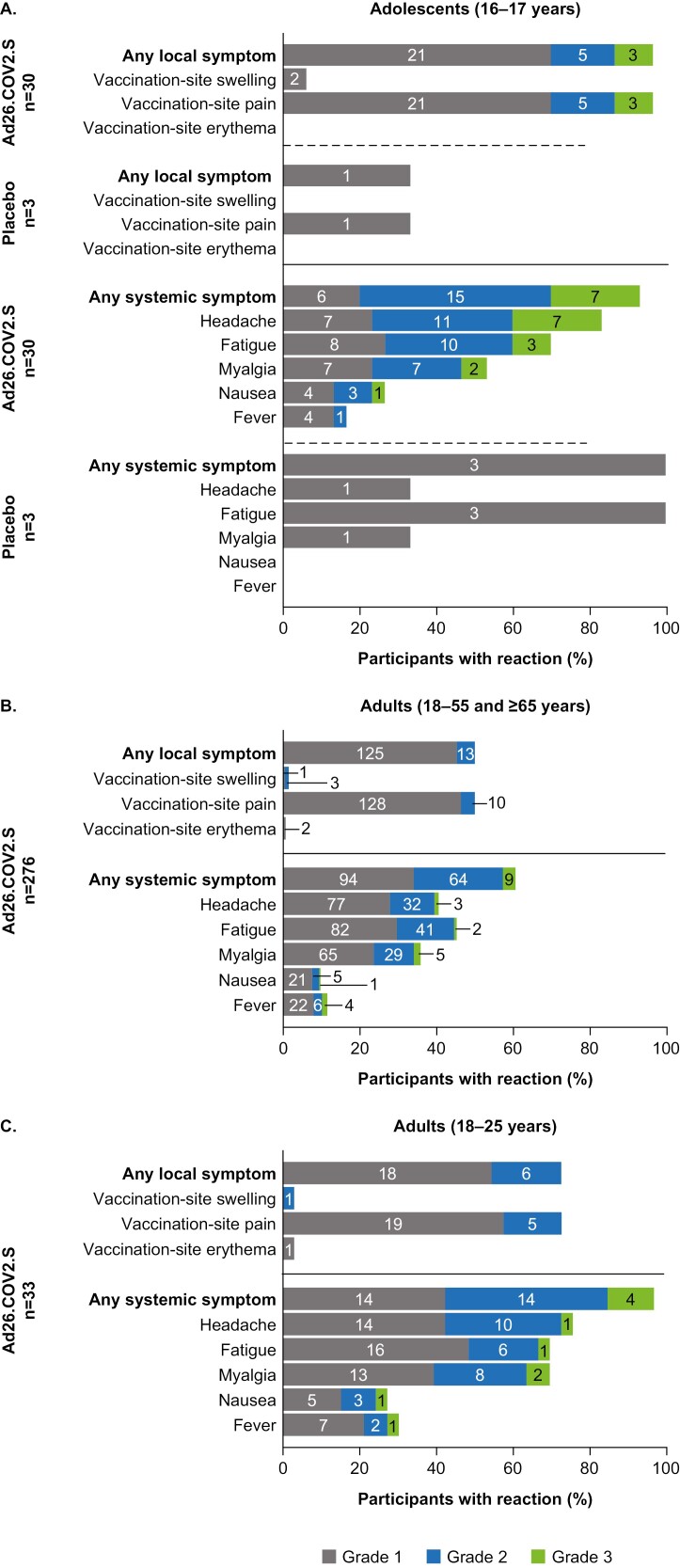
Figure 1.
Solicited adverse events in (A) adolescent participants who received 2.5 × 1010 vp Ad26.COV2.S vaccine or placebo; (B) healthy adults aged 18–55 and ≥65 years who received 5 × 1010 vp Ad26.COV2.S vaccine; and (C) healthy adults aged 18–25 years who received 5 × 1010 vp Ad26.COV2.S vaccine. The number of participants reporting each grade is shown within each section of bars. Abbreviations: vp, viral particles.