Fig. 2.
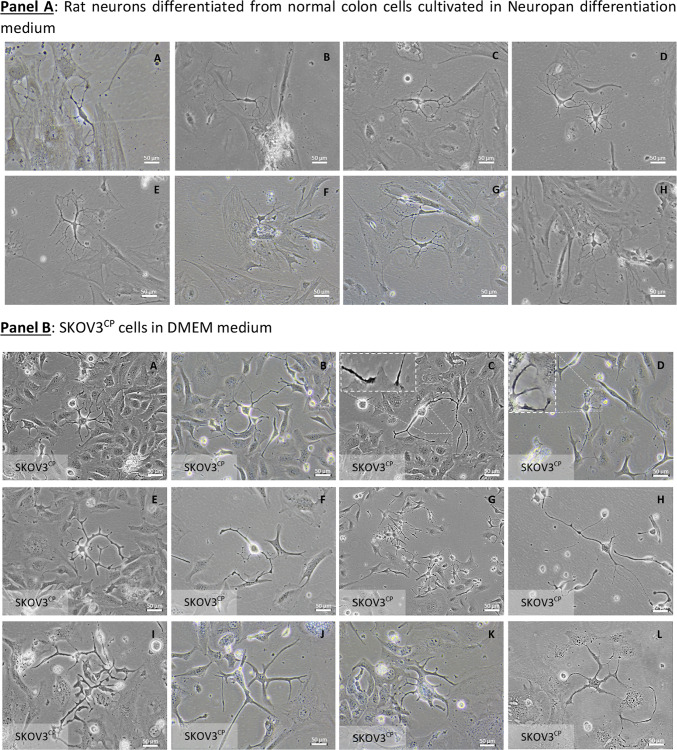
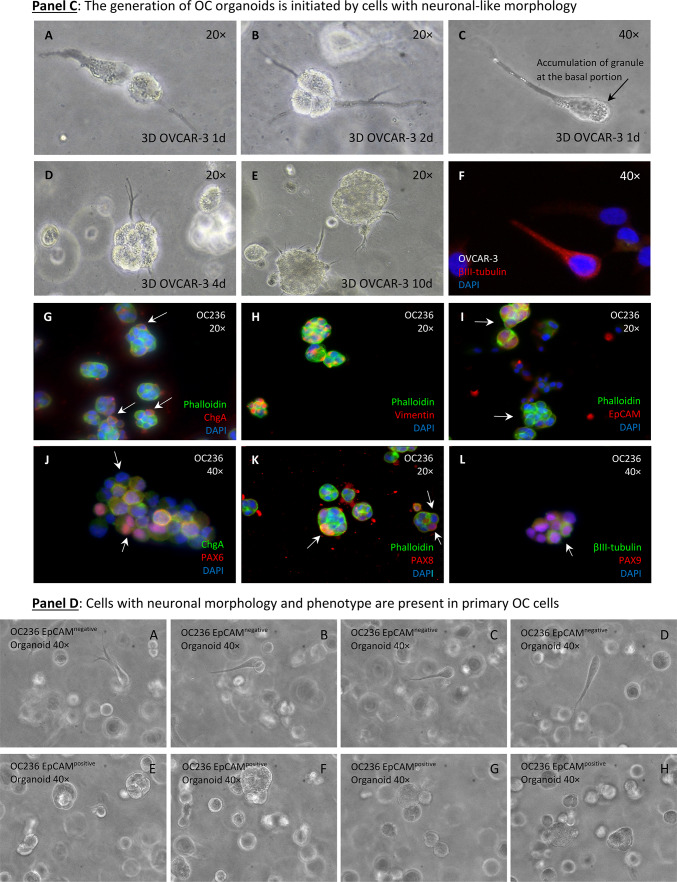
Communication networks and differentiation of cells in 2D and 3D cell cultures. Panel A: normal enteric neurons isolated from Wistar rats interacting with adjacent cells via neuron-somatic cell (A, B, C, E, F & H) or neuron-neuron (D & G) communication, with the contact being mediated by dendrite-like structures. Panel B: cell–cell communication among SKOV3CP cells similar to normal tissue (neuron-like somatic cells: close-up from pictures C & D; neuron-neuron, H) via dendrite-like protoplasmic extensions. Panel C: culture of primary OC cells in 3D Geltrex gel supplemented with organoid medium. The clusters of OC cells maintained in organoid conditions revealed that in the early stages, these cells develop a neuronal appearance similar to 2D models (A-E). In this context, we observed cells with a morphology resembling colonic neuroendocrine cells of the crypts characterized by the typical basal granule accumulation (C). The neuronal nature of these cells is confirmed by the expression of βIII-tubulin (F). In 3D cultures, we observed the recapitulation of ChgA expressing cells (G, white arrows). Vimentin was widely expressed in these cultures (H), while EpCAM was differentially expressed among cell clusters (I, white arrows). The transcription factors PAX6, PAX8 and PAX9 were differentially expressed by cells within the organoids (J-L, white arrows). Panel D: Primary ovarian carcinoma 3D cell cultures with neuronal morphology and phenotype. OC236 subpopulations grow in nest-like forms with EpCAMnegative cells sorted from the EpCAMpositive fraction using immunomagnetic MicroBeads. In organoid cultures derived from EpCAMnegative fraction, cells with a neuronal phenotype, similar to those observed in 2D cell cultures (Panel C, A-C) appeared. Contrarily, in EpCAMpositive cells this phenotype is rare to find (Panel D, E–H). Of note is the tendency of EpCAMpositive cells to generate clusters similar to organoids. Pictures are representative of several experiments. Magnification is reflected in each picture.