Abstract
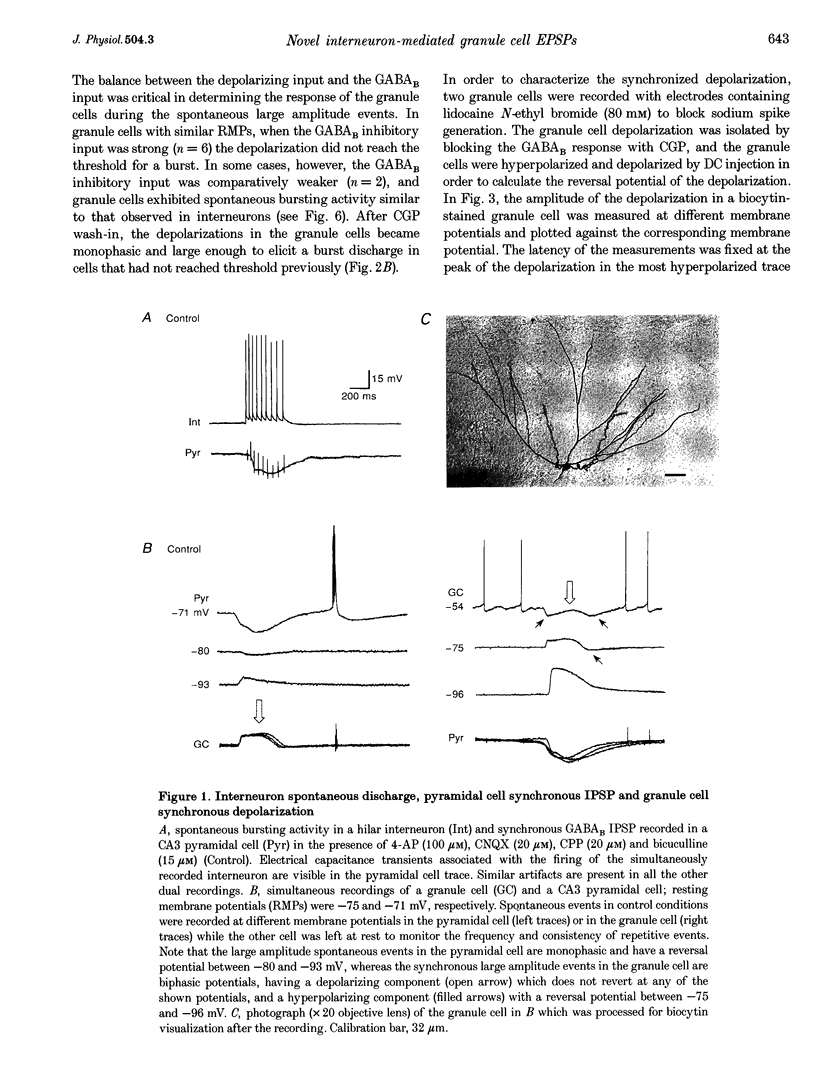
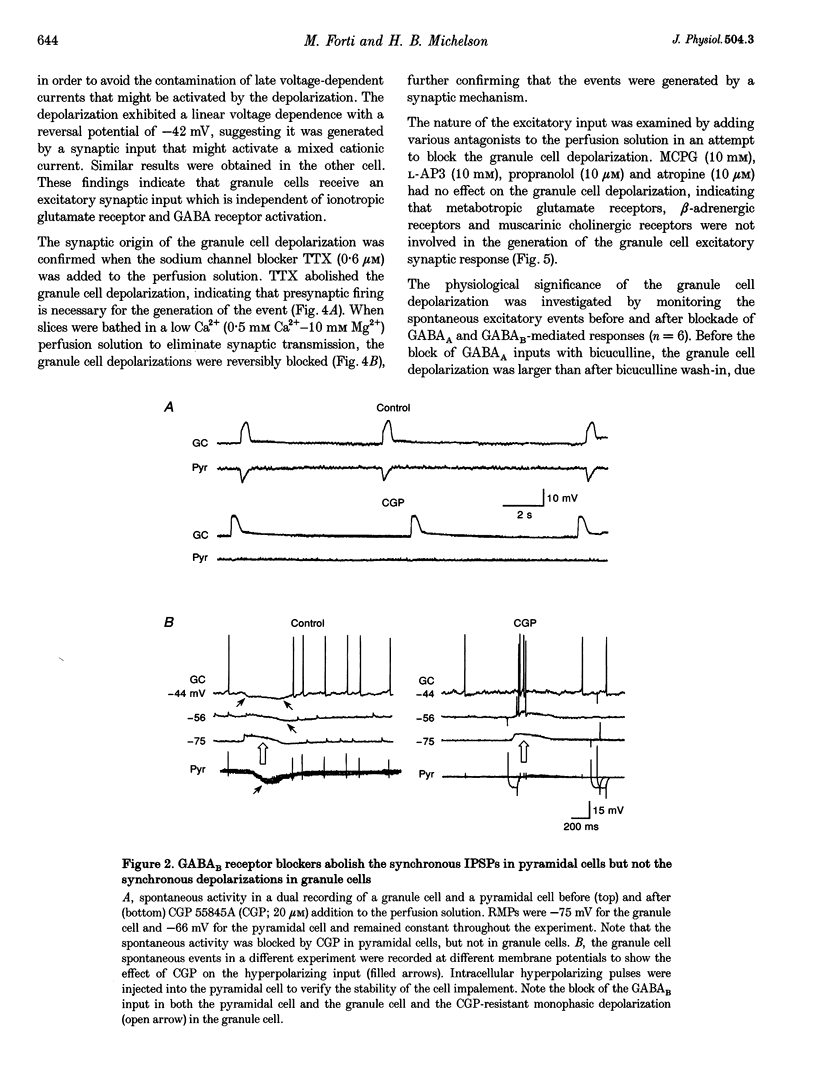
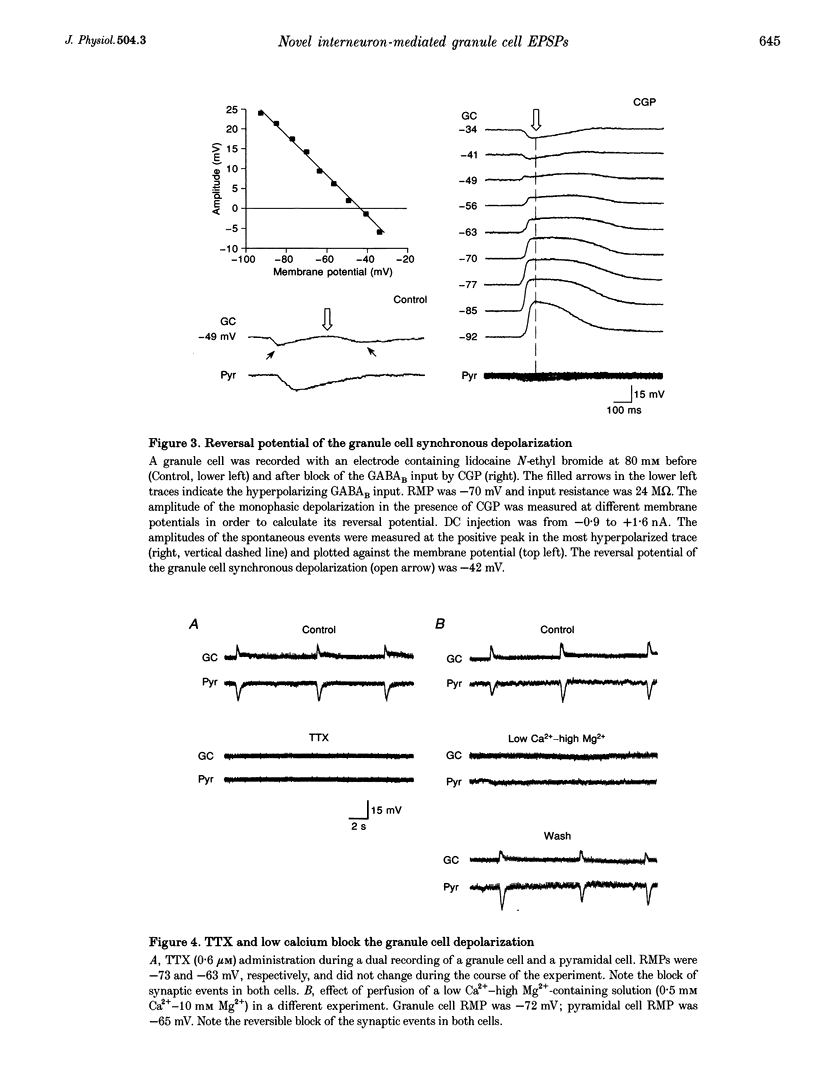
1. Dual intracellular recordings of granule cells, hilar interneurons and CA3 pyramidal cells were performed in transverse slices of guinea-pig hippocampus. At resting membrane potential, in the presence of 4-aminopyridine, ionotropic glutamate receptor antagonists and the GABAA receptor antagonist bicuculline, granule cells showed spontaneous, large amplitude depolarizations correlated with synchronous bursting activity of interneurons. 2. Under these conditions, pyramidal cells exhibited large amplitude monophasic GABAB inhibitory postsynaptic potentials (IPSPs) synchronous with the GABAergic interneuron burst discharges. The granule cells also received a GABAB input, which was evident only when the neurons were depolarized by DC injection. The GABAB receptor antagonist CGP 55,845A (CGP) blocked the GABAB IPSPs in both pyramidal cells and granule cells; however, the depolarizing potential in granule cells was unaffected by the drug. 3. The granule cells depolarization in the presence of CGP was monophasic and exhibited linear voltage dependence with a reversal potential around -40 mV, suggesting that it was generated by a synaptic input activating a mixed cationic current. 4. The granule cell depolarization was abolished following the addition of tetrodotoxin to the bath. In addition, perfusing the slice with a low Ca(2+)-containing solution (0.5 mM Ca(2+)-10 mM Mg2+) also abolished the granule cell depolarization, confirming the synaptic origin of the event. 5. (S)-Methyl-4-carboxyphenylglycine, L-(+)-2-amino-3-phosphonopropionic acid, propranolol and atropine did not affect the granule cell depolarization, indicating that metabotropic glutamate receptors, beta-adrenergic receptors and muscarinic cholinergic receptors were not involved in generating the granule cell depolarizing synaptic response. 6. These findings indicate that, in the absence of both glutamatergic and GABAergic inputs, synchronous interneuronal activity can produce a depolarizing synaptic response in granule cells. The neurochemical responsible for the depolarization is currently under investigation.
Full text
PDF



Images in this article
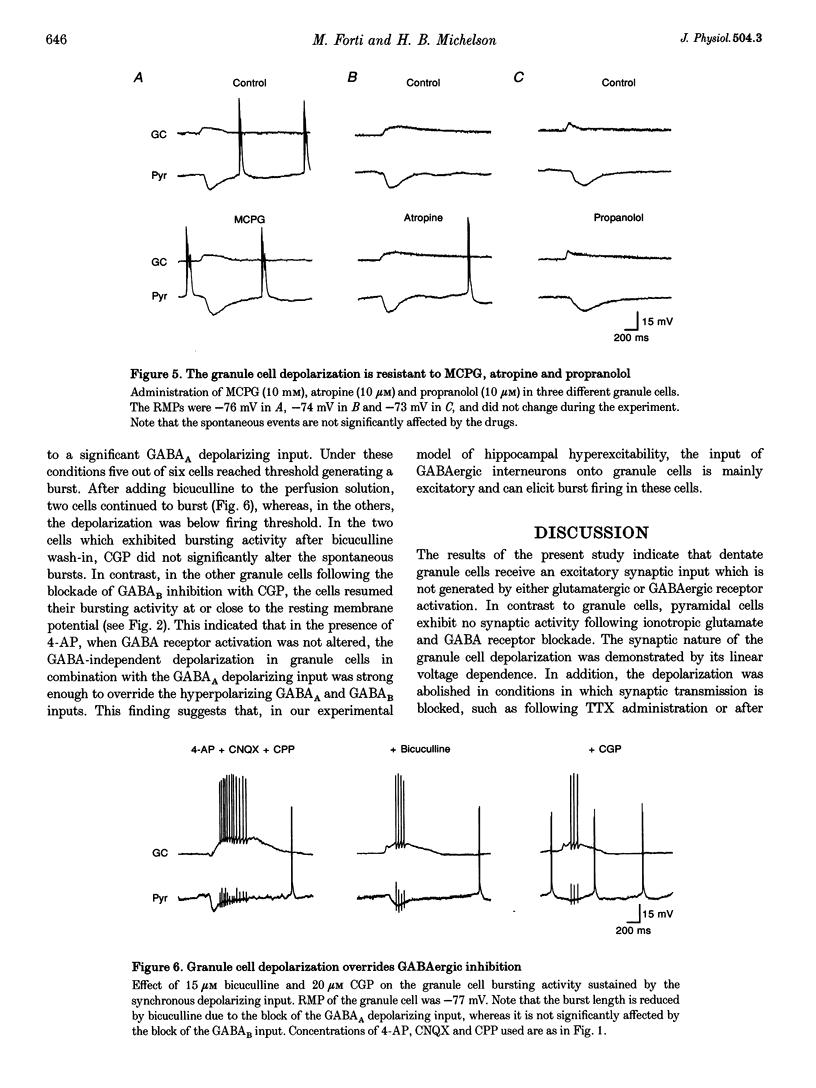
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978 Dec 15;182(4 Pt 2):851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Amaral D. G., Witter M. P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Aram J. A., Michelson H. B., Wong R. K. Synchronized GABAergic IPSPs recorded in the neocortex after blockade of synaptic transmission mediated by excitatory amino acids. J Neurophysiol. 1991 May;65(5):1034–1041. doi: 10.1152/jn.1991.65.5.1034. [DOI] [PubMed] [Google Scholar]
- Collins R. C., Tearse R. G., Lothman E. W. Functional anatomy of limbic seizures: focal discharges from medial entorhinal cortex in rat. Brain Res. 1983 Nov 28;280(1):25–40. doi: 10.1016/0006-8993(83)91170-8. [DOI] [PubMed] [Google Scholar]
- Dudek F. E., Deadwyler S. A., Cotman C. W., Lynch G. Intracellular responses from granule cell layer in slices of rat hippocampus: perforant path synapse. J Neurophysiol. 1976 Mar;39(2):384–393. doi: 10.1152/jn.1976.39.2.384. [DOI] [PubMed] [Google Scholar]
- Fournier E., Crepel F. Electrophysiological properties of dentate granule cells in mouse hippocampal slices maintained in vitro. Brain Res. 1984 Oct 8;311(1):75–86. doi: 10.1016/0006-8993(84)91400-8. [DOI] [PubMed] [Google Scholar]
- Michelson H. B., Wong R. K. Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science. 1991 Sep 20;253(5026):1420–1423. doi: 10.1126/science.1654594. [DOI] [PubMed] [Google Scholar]
- Michelson H. B., Wong R. K. Synchronization of inhibitory neurones in the guinea-pig hippocampus in vitro. J Physiol. 1994 May 15;477(Pt 1):35–45. doi: 10.1113/jphysiol.1994.sp020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan E., Stringer J. L. Burst characteristics of dentate gyrus granule cells: evidence for endogenous and nonsynaptic properties. J Neurophysiol. 1996 Jan;75(1):124–132. doi: 10.1152/jn.1996.75.1.124. [DOI] [PubMed] [Google Scholar]
- Paré D., deCurtis M., Llinás R. Role of the hippocampal-entorhinal loop in temporal lobe epilepsy: extra- and intracellular study in the isolated guinea pig brain in vitro. J Neurosci. 1992 May;12(5):1867–1881. doi: 10.1523/JNEUROSCI.12-05-01867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H. E. EPSPs of dentate gyrus granule cells during epileptiform bursts of dentate hilar "mossy" cells and area CA3 pyramidal cells in disinhibited rat hippocampal slices. J Neurosci. 1994 Oct;14(10):6041–6057. doi: 10.1523/JNEUROSCI.14-10-06041.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer J. S., Williamson A. Relationship between synaptic activity and prolonged field bursts in the dentate gyrus of the rat hippocampal slice. J Neurophysiol. 1995 Nov;74(5):1947–1952. doi: 10.1152/jn.1995.74.5.1947. [DOI] [PubMed] [Google Scholar]
- Strata F., Atzori M., Molnar M., Ugolini G., Tempia F., Cherubini E. A pacemaker current in dye-coupled hilar interneurons contributes to the generation of giant GABAergic potentials in developing hippocampus. J Neurosci. 1997 Feb 15;17(4):1435–1446. doi: 10.1523/JNEUROSCI.17-04-01435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. L., Lothman E. W. Maximal dentate gyrus activation: characteristics and alterations after repeated seizures. J Neurophysiol. 1989 Jul;62(1):136–143. doi: 10.1152/jn.1989.62.1.136. [DOI] [PubMed] [Google Scholar]
- Stringer J. L., Williamson J. M., Lothman E. W. Induction of paroxysmal discharges in the dentate gyrus: frequency dependence and relationship to afterdischarge production. J Neurophysiol. 1989 Jul;62(1):126–135. doi: 10.1152/jn.1989.62.1.126. [DOI] [PubMed] [Google Scholar]