Abstract
Yeo’s index, the product of the mitral leaflet separation index and dimensionless index of mitral valve (MV), was recently described to accurately identify severe rheumatic mitral stenosis (MS). We assess the association between Yeo’s index and clinical outcomes in patients with rheumatic MS. We studied 297 patients with rheumatic MS. Clinical and echocardiographic data were obtained from the electronic medical record and Yeo’s index was measured in all cases. The outcome studied was a composite of all cause death, heart failure (HF) hospitalisation, MV intervention and stroke or transient ischaemic attack. We also performed subgroup analysis of patients without pre-existing atrial fibrillation (AF) to assess for association with new onset AF. The median follow up was 6.3 years; 145 patients (48.8%) developed the composite outcome. Yeo’s index (p < 0.001), mitral valve area (MVA) by pressure half-time (PHT) (p = 0.028) and planimetry (p < 0.001), age (p = 0.016), history of diabetes mellitus (p = 0.029), previous HF (p = 0.021), left ventricular ejection fraction (p = 0.022), and pulmonary artery systolic pressure (p = 0.007) were univariately associated with the composite outcome. Yeo’s index remained independently associated with the composite outcome in multivariate analysis (p < 0.001, HR 0.094, 95% CI 0.260–0.340). This was primarily driven by MV intervention. In a subgroup analysis of patients without pre-existing AF, Yeo’s index was independently associated with new onset AF (p = 0.024, HR 0.354, 95% CI 0.143–0.874). This demonstrated that Yeo’s index was independently associated with clinical outcomes in patients with rheumatic MS which was mainly driven by MV intervention.
Keywords: Mitral stenosis, Rheumatic heart disease, Yeo’s index
Subject terms: Valvular disease, Heart failure
Introduction
Mitral stenosis (MS) is predominantly associated with rheumatic heart disease1,2. While degenerative calcific MS is becoming more common in the developed world, rheumatic MS remains the leading aetiology of MS and confers a significant disease burden2,3. In rheumatic MS, the inflammatory rheumatic process results in progressive fibrosis of the valve leaflets and commissural fusion amongst other structural changes, leading to a diminished mitral valve area (MVA) and impaired diastolic flow across the mitral valve (MV)4,5. As MS becomes more severe, progressive increase in left atrial pressure leads to left atrial dilatation and the development of atrial fibrillation (AF), pulmonary congestion and pulmonary hypertension due to pulmonary vascular remodelling.
Echocardiography remains the main tool for assessment of the severity of MS through measurement of the MVA, which is typically obtained through two-dimensional (2D) planimetry or pressure half time (PHT) method. Other ways to measure MVA include the continuity equation (CE) and proximal isovelocity surface area methods3,6–8. Each method has its own limitations and current guidelines recommend the use of a multi-parametric approach to assess the severity of MS9,10. Despite well-established indications for MV intervention, relatively little is known about the predictors of outcomes in patients with rheumatic MS. It is also recognised that predicting progression of MS is challenging with high variability in the rate of progression between different patients11.
Recently, our group described a novel index, termed Yeo’s index, which demonstrated good performance for identifying severe rheumatic MS when compared to existing measures of MVA12. Yeo’s index is the product of the mitral leaflet separation index (MLSI) and the MV dimensionless index (DI). MV DI is derived by dividing left ventricular outflow tract (LVOT) time-velocity integral (TVI) by MV TVI12. We found that Yeo’s index ≤ 0.26 cm accurately identified patients with severe MS (≤ 1.5cm2), while Yeo’s index ≤ 0.15 cm accurately identified very severe MS (≤ 1.0cm2). In this study, we sought to determine whether Yeo’s index is associated with clinical outcomes in patients with rheumatic MS.
Methods
We studied a retrospective cohort of 297 cases of rheumatic MS who underwent transthoracic echocardiography (TTE) at our academic medical centre, the National University Heart Centre Singapore. We excluded patients with degenerative MS. Echocardiographic data was obtained from our centre’s database and we reviewed the electronic medical records for pertinent clinical information and clinical outcomes. The research protocol was approved by our centre’s Institutional Review Board and the study conforms to the ethical principles laid out in the 1975 Declaration of Helsinki.
Yeo’s index was measured by operators (RL, TL and TCY) who were blinded to the clinical, echocardiographic (including MVA measurements) and outcome data. MLSI was measured by taking the mean of the maximal diastolic separation of the MV leaflet tips in the parasternal long-axis and apical four-chamber views as described previously13. MV DI was derived by dividing LVOT TVI by MV TVI14. Yeo’s Index was then derived by multiplying MLSI by MV DI12. When the patient was in sinus rhythm, measurements were taken across 3 cardiac cycles; whereas measurements from 5 cardiac cycles were used when the rhythm was in AF. Intra- and inter-observer variability of Yeo’s index was assessed by intra-class correlation coefficients (ICC) with 95% confidence intervals using SPSS reliability analyses15.
Outcomes including all cause death, heart failure (HF) hospitalisation, MV intervention, and stroke or transient ischaemic attack (TIA) were collected from the electronic medical records. A composite of these four outcomes was studied. For the endpoint of MV intervention, we only considered MV interventions that were performed for the primary indication of significant MS. Patients who had concomitant MV intervention when undergoing surgery for another indication such as surgery for significant mitral regurgitation or aortic valve disease or coronary artery bypass graft surgery were excluded. These patients were still included in the analysis for all cause death, heart failure hospitalization and stroke or TIA. Regression analysis using the Cox proportional hazards model was then employed to assess associations between variables and the studied outcomes. We systematically analysed all clinical variables including age, sex, co-morbidities etc. as well as echocardiographic variables using univariate Cox regression analysis against the composite outcome. Having identified variables which were significant on univariate analysis, we then used these variables to construct a multivariate Cox regression model together with age, sex and Yeo’s index. Age and sex were selected on the basis of being biologically significant variables, while Yeo’s index was the variable of interest in this study. The potential for multicollinearity was assessed by examining the correlation matrix generated using all co-variates employed in this multivariate Cox regression model, and did not demonstrate significant correlation between individual co-variates. To provide further insights into the prognostic value of Yeo’s index, we compared the composite outcomes in patients included in the following subgroups:
Yeo’s index ≤ 0.26 cm (indicating severe MS) versus MVA by PHT > 1.5 cm2
Yeo’s index ≤ 0.26 cm (indicating severe MS) versus MVA by planimetry > 1.5 cm2
Yeo’s index > 0.26 cm (indicating non severe MS) versus MVA by PHT ≤ 1.5 cm2
Yeo’s index > 0.26 cm (indicating non severe MS) versus MVA by planimetry ≤ 1.5 cm2
Yeo’s index was next assessed for association with the individual outcomes univariately followed by using multivariate analysis. New onset AF was identified as a clinical outcome of interest in our cohort with rheumatic MS. However, as many patients in the study cohort had pre-existing AF at the time of their MS diagnosis, it was not possible to incorporate new-onset AF into the composite outcome for the study cohort. Therefore, we conducted a subgroup analysis in patients without pre-existing AF for the presence of new onset AF. We performed univariate and multivariate analysis of all clinical and echocardiographic variables in these patients to assess for association with new onset AF.
Categorical variables were presented as frequency and percentages. Continuous variables were assessed for normality using the Shapiro–Wilk test and descriptive statistics presented as mean ± standard deviation in the event of normally distributed data, or median and interquartile range in the event that data were not normally distributed. P-values less than 0.05 were deemed statistically significant. Ninety-five percent confidence intervals (CI) are presented where relevant. Differences in survival analysis were considered statistically significant if there was no overlap in the 95% confidence intervals of the mean duration of survival. Statistical analysis was performed with IBM SPSS Statistics Version 23 (IBM Corp., Armonk, NY, USA) as well as MedCalc (MedCalc Software Ltd, Belgium).
Results
The study cohort included more females (212 patients, 71.4%) and relatively older patients with a mean age of 55.6 ± 13.4 years compared to historical cohorts with rheumatic MS16. Over half had pre-existing AF (167 patients, 56.2%) while 39 patients (13.1%) had a previous history of stroke or TIA, and 56 patients (18.9%) had a history of HF at the time of the index echocardiogram. Further demographic and clinical data for the study cohort are provided in Table 1.
Table 1.
Baseline demographic and clinical data for the study cohort.
| Clinical variable | Overall (n = 297) | |
|---|---|---|
| Number (percentage), mean value (± 1 standard deviation), or median (interquartile range) | ||
| Age (years) | 55.6 (± 13.4) | |
| Female sex | 212 (71.4%) | |
| Ethnicity | Chinese | 171 (57.6%) |
| Malay | 64 (21.5%) | |
| Indian | 18 (6.1%) | |
| Other ethnicities | 49 (14.8%) | |
| Height (cm) | 158.0 (152.0–163.0) | |
| Weight (kg) | 60.0 (51.5–68.0) | |
| BMI (kg/m2) | 25.1 (21.0–27.2) | |
| BSA (m2) | 1.62 (1.49–1.74) | |
| Blood pressure (mmHg) | 126 (110–140) / 70 (62–79) | |
| Heart rate (beats per minute) | 75 (64–87) | |
| Hypertension | 123 (41.4%) | |
| Hyperlipidemia | 111 (37.4%) | |
| Diabetes mellitus | 77 (25.9%) | |
| Ischaemic heart disease | 42 (14.1%) | |
| Stroke or transient ischaemic attack | 39 (13.1%) | |
| Atrial fibrillation | 167 (56.2%) | |
| Heart failure | 56 (18.9%) | |
| Chronic kidney disease | 29 (9.8%) | |
| Peripheral vascular disease | 9 (3.0%) | |
| Asthma or COPD | 23 (7.7%) | |
| Antiplatelet | 85 (28.6%) | |
| Anticoagulation | 140 (47.1%) | |
| Beta-blocker | 153 (51.5%) | |
| Calcium-channel-blocker | 34 (11.4%) | |
| Diuretic | 85 (28.6%) | |
| ACE-I/ARB | 79 (26.6%) | |
| MRA | 6 (2.0%) | |
| Digoxin | 87 (29.3%) | |
| Statin | 119 (40.1%) | |
ACE-I; angiotensin converting enzyme-inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease; MRA, mineralocorticoid receptor antagonist.
Table 2 shows the echocardiographic parameters for the study patients. Two hundred and one (67.7%) patients had isolated rheumatic MS, while 96 (32.3%) had mixed valve disease, defined as presence of MR and/or aortic valve disease of greater than mild severity. Of the patients with mixed valve disease, 67 (22.6%) had MR, 24 (8.1%) had aortic stenosis (AS), and 5 (1.7%) had both AS and MR. The mean MVA was 1.45 ± 0.49 cm2 by two-dimensional planimetry and 1.54 ± 0.46 cm2 by PHT. One hundred and thirty one (44.1%) patients had severe MS (MVA ≤ 1.5 cm2) according to both the planimetry and PHT measurements. There was no significant difference in MVA between patients with a history of HF compared to those without HF (MVA by PHT, 1.46 ± 0.54 cm2 for patients with prior HF versus 1.56 ± 0.57 cm2 for patients without HF, p = 0.212; MVA by planimetry, 1.39 ± 0.44 cm2 for patients with HF versus 1.46 ± 0.50 cm2 for patients without HF, p = 0.372). The intra-observer and inter-observer variabilities for Yeo’s index as assessed by the intraclass correlation coefficients (r) were 0.98 (95% CI 0.90–0.99) and 0.96 (95% CI 0.86–0.98), respectively.
Table 2.
Baseline echocardiographic parameters for the study cohort.
| Echocardiographic parameters | Overall (n = 297) |
|---|---|
| Median (interquartile range) | |
| Left atrial diameter (mm) | 50.0 (45.0–55.0) |
| Left atrial volume (ml) | 94.3 (70.5–125.5) |
| Left atrial volume index (ml/m2) | 58.8 (43.5–78.5) |
| Left ventricle mass index (g/m2) | 86.0 (69.0–110.5) |
| Left ventricle end diastolic volume (ml) | 97.0 (79.0–118.0) |
| Left ventricle end systolic volume (ml) | 35.0 (27.0–51.0) |
| Left ventricle stroke volume (ml) | 59.0 (45.0–73.0) |
| Left ventricle ejection fraction (%) | 60.0 (53.5–65.0) |
| Left ventricle outflow tract diameter (mm) | 20.0 (18.0–21.0) |
| Left ventricle outflow tract pulsed-wave TVI (cm) | 18.0 (15.0–21.0) |
| Heart rate during echocardiographic study (bpm) | 75 (62–87) |
| Estimated cardiac output (L/min) | 4.10 (3.32–5.12) |
| Estimated cardiac index (L/min/m2) | 2.49 (2.06–3.06) |
| Mitral valve continuous-wave TVI (cm) | 53.0 (43.1–65.6) |
| Pulmonary artery systolic pressure (mmHg) | 42.0 (35.0–57.0) |
| MVA by two-dimensional planimetry (cm2) | 1.40 (1.10–1.80) |
| MVA by pressure half-time (cm2) | 1.49 (1.12–1.82) |
| Transmitral mean pressure gradient (mmHg) | 6.21 (4.5–9.0) |
| Mitral valve DI | 0.341 (0.257–0.420) |
| Mitral leaflet separation index | 0.83 (0.67–1.02) |
| Yeo’s Index | 0.27 (0.19–0.41) |
DI, dimensionless index; MVA, mitral valve area; TVI, time-velocity integral.
The median follow up was 6.3 years. One hundred and forty five patients (48.8%) developed the composite outcome: death in 90 patients (30.3%), 39 patients (13.1%) had HF hospitalisation, 50 patients (16.8%) underwent MV intervention, and 28 patients (9.4%) had a stroke or TIA. Of the 50 patients who underwent MV intervention, 22 patients (44.0%) had percutaneous transvalvular mitral commissurotomy, 26 patients underwent surgical MV replacement (52.0%) and 2 patients (4.0%) had an open mitral valvotomy. One patient was excluded from analysis of MV intervention as the patient had intervention primarily for the indication of symptomatic critical AS and had concomitant MV surgery for moderate MS.
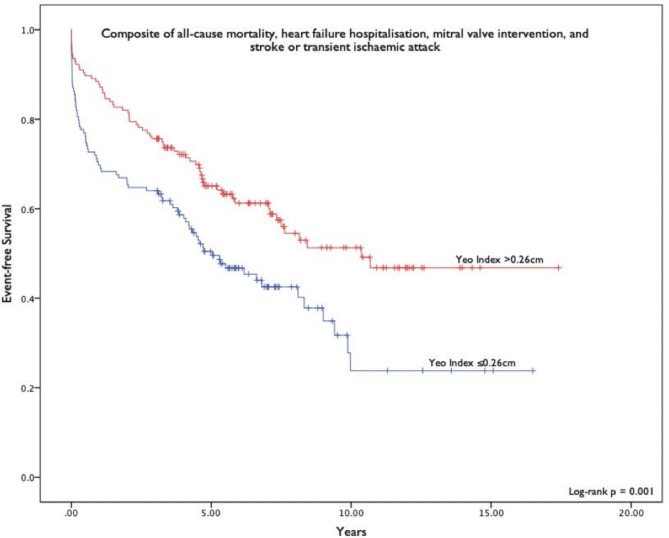
For the composite outcome, univariate analysis showed that age, history of diabetes mellitus (DM), history of HF, Yeo’s index, MVA by PHT and planimetry, left ventricular ejection fraction (LVEF), and pulmonary artery systolic pressure (PASP) had significant association. Other clinical variables including pre-existing AF, history of ischaemic heart disease or stroke or TIA and other relevant echocardiographic variables such as the left atrial volume index (LAVI) and mean transmitral pressure gradient were not univariately associated with the composite outcome. Table 3 shows the variables which demonstrated significant association with the composite outcome on univariate analysis. Figure 1 shows the event free survival of Yeo’s index ≤ 0.26 cm (indicating MVA ≤ 1.5cm2) versus Yeo index > 0.26 cm.
Table 3.
Univariate Cox regression analysis for the composite outcomes of all cause death, heart failure hospitalisation, mitral valve intervention, and stroke or transient ischaemic attack.
| Variable | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Age (year) | 1.016 (1.003–1.028) | 0.016 |
| History of diabetes mellitus | 0.679 (0.479–0.962) | 0.029 |
| History of heart failure | 1.578 (1.073–2.323) | 0.021 |
| LVEF (%) | 0.982 (0.967–0.997) | 0.022 |
| PASP (mmHg) | 1.012 (1.003–1.021) | 0.007 |
| MVA by PHT (cm2) | 0.684 (0.488–0.959) | 0.028 |
| MVA by Planimetry (cm2) | 0.499 (0.341–0.732) | < 0.001 |
| Yeo’s index (cm) | 0.099 (0.033–0.300) | < 0.001 |
CI, confidence interval; LVEF, left ventricular ejection fraction; MVA, mitral valve area; PASP, pulmonary artery systolic pressure; PHT, pressure half-time.
Fig. 1.
Kaplan–Meier curves comparing event free survival of patients with Yeo’s index ≤ 0.26 cm versus those with survival > 0.26 cm for the composite clinical outcome.
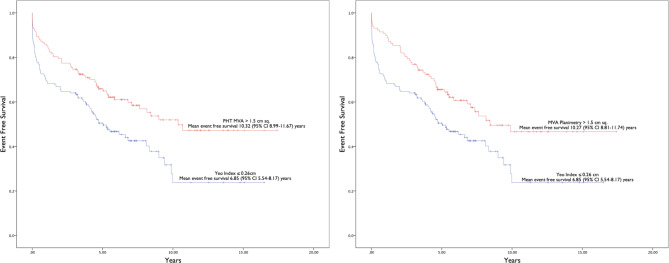
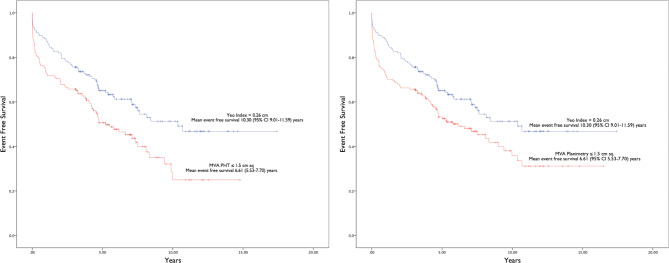
The result of multivariate Cox regression analysis for Yeo’s index is presented in Table 4. Only age and Yeo’s index were independently associated with the composite outcomes. Figure 2 shows that the event free survival of patients with Yeo’s index ≤ 0.26 cm was significantly worse than the event free survival of patients with MVA by PHT > 1.5cm2 and MVA by planimetry > 1.5cm2 respectively (mean event free survival 6.85 years, 95% CI 5.54–6.17 versus mean event free survival 10.32 years, 95% CI 8.99–11.67 and 10.27 years, 95% CI 8.81–11.74 respectively). On the other hand, Fig. 3 shows that the event free survival of patients with Yeo’s index > 0.26 cm was significantly better than the event free survival of patients with MVA by PHT ≤ 1.5cm2 and MVA by planimetry ≤ 1.5cm2 respectively (mean event free survival 10.30 years, 95% CI 9.01–11.59 versus mean event free survival 6.61 years, 95% CI 5.53–7.70 and 7.68 years, 95% CI 6.52–8.17 respectively). We found that there was no significant difference in the mean event free survival between patients identified as non severe MS by Yeo’s index (> 0.26 cm) and those identified as non severe MS by PHT or planimetry (MVA > 1.5cm2). Similarly, there was no difference in mean event free survival between patients identified as severe MS by Yeo’s index (≤ 0.26 cm) and those identified as severe MS by PHT (MVA ≤ 1.5cm2). The mean event free survival of patients identified as severe MS by Yeo’s index (≤ 0.26 cm) was poorer than those identified as severe MS by planimetry (MVA ≤ 1.5cm2).
Table 4.
Multivariate Cox regression analysis incorporating Yeo’s index for the composite outcomes of all cause death, heart failure hospitalisation, mitral valve intervention, and stroke or transient ischaemic attack.
| Multivariate analysis: variables and statistical parameters | ||
|---|---|---|
| Variable | Hazard ratio (95% CI) | p-value |
| Age (year) | 1.018 (1.003–1.033) | 0.019 |
| Female sex | 0.959 (0.643–1.432) | 0.839 |
| History of diabetes mellitus | 0.693 (0.462–1.041) | 0.077 |
| History of heart failure | 1.336 (0.867–2.060) | 0.190 |
| LVEF (%) | 0.997 (0.979–1.015) | 0.726 |
| PASP (mmHg) | 1.009 (0.999–1.019) | 0.083 |
| Yeo’s index (cm) | 0.094 (0.026–0.340) | < 0.001 |
CI, confidence interval; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure.
Fig. 2.
Kaplan Meier curves comparing event free survival of patients with Yeo’s index ≤ 0.26 cm versus mitral valve area by pressure half-time and planimetry > 1.5 cm2 for the composite clinical outcome. Abbreviations: CI, confidence interval; MVA, mitral valve area; PHT, pressure half-time.
Fig. 3.
Kaplan Meier curves comparing event free survival of patients with Yeo’s index > 0.26 cm versus mitral valve area by pressure half-time and planimetry ≤ 1.5 cm2 for the composite clinical outcome. Abbreviations: CI, confidence interval; MVA, mitral valve area; PHT, pressure half-time.
Multivariate analysis for individual clinical outcomes showed that Yeo’s index was only independently associated with MV intervention (p < 0.001). It has borderline significant association with all cause death (p = 0.052) and was not independently associated with HF hospitalisation or stroke or TIA.
In the subgroup analysis of patients without pre-existing AF (n = 130 patients), Yeo’s index (p = 0.048), LAVI (p = 0.002) and PASP (p = 0.002) were univariately associated with new onset AF. MVA by PHT, MVA by planimetry, mean transmitral pressure gradient, LVEF, age, sex and other echocardiographic and clinical variables were not significantly associated with new onset AF. In multivariate analysis including age, sex and variables that were univariately significant, Yeo’s index remained independently associated with new onset AF (p = 0.024). Table 5 shows the subgroup analysis for new onset AF. If sex was excluded from multivariate analysis, the results are as follows: age (year) p = 0.007, HR = 1.043; Yeo’s index (non severe in comparison to severe) p = 0.014, HR = 0.330; LAVI p = 0.002, HR = 1.017; PASP p = NS, HR = 1.018. If both age and sex were excluded, the remaining results are: Yeo’s index (non severe in comparison to severe) p = 0.070 (NS), HR = 0.450; LAVI p = 0.035, HR = 1.009; PASP p = NS; HR = 1.014.
Table 5.
Subgroup Cox regression analysis for patients in sinus rhythm at baseline for the outcome of new onset atrial fibrillation.
| Univariate analysis: significant variables | ||
|---|---|---|
| Variable | Hazard ratio (95% CI) | p-value |
| Yeo Index (non-severe versus severe) | 0.478 (0.230–0.993) | 0.048 |
| LAVI (cm3/m2) | 1.011 (1.004–1.017) | 0.002 |
| PASP (mmHg) | 1.025 (1.009–1.042) | 0.002 |
| Multivariate analysis | ||
| Age (year) | 1.045 (1.013–1.078) | 0.006 |
| Female sex | 0.717 (0.296–1.740) | 0.462 |
| LAVI (cm3/m2) | 1.017 (1.006–1.028) | 0.002 |
| PASP (mmHg) | 1.018 (0.998–1.040) | 0.080 |
| Yeo Index (non-severe versus severe) | 0.354 (0.143–0.874) | 0.024 |
CI, confidence interval; LAVI, left atrial volume index; PASP, pulmonary artery systolic pressure.
Discussion
Our group recently proposed Yeo’s index as a complementary tool to existing measures of MVA for the assessment of severity of MS. We found that Yeo’s index ≤ 0.26 cm accurately identified patients with severe MS (≤ 1.5cm2), while Yeo’s index ≤ 0.15 cm accurately identified very severe MS (≤ 1.0cm2)12. Existing measures of MS severity either quantify the anatomic severity of the disease (MVA by planimetry) or the functional consequences of the stenotic MV orifice (MVA by PHT and transmitral mean pressure gradient). Conceptually, Yeo’s index, derived by the product of the MLSI and the MV DI, might be better as it combines both anatomic and functional assessment in a single parameter. MV DI was used instead of mean transmitral pressure gradient as it is less flow dependent. However, the association between Yeo’s index and clinical outcomes has not yet been demonstrated.
The results of the present study show that in patients with rheumatic MS, Yeo’s index was independently associated with the composite outcome of all cause death, heart failure hospitalisation, MV intervention and stroke or TIA. This was primarily driven by its association with MV intervention while it has only borderline significant association with all cause death. Subgroup analysis further informed the prognostic value of Yeo’s index by showing that the event free survival of patients with Yeo’s index ≤ 0.26 cm (indicating severe MS) was significantly worse than the event free survival of patients with non-severe MS as assessed by traditional measures of MS severity using MVA by PHT and planimetry. On the other hand, the event free survival of patients with Yeo’s index > 0.26 cm (indicating non severe MS) was significantly better than the patients with severe MS as assessed by traditional measures of MS severity using MVA by PHT and planimetry. In the subgroup analysis of patients without pre-existing AF, Yeo’s index was found to be independently associated with new onset AF.
The natural history of rheumatic MS has been described by historical observational cohorts from as early as 1960, which chiefly established poor functional status due to heart failure and the presence of AF as factors associated with worse clinical outcomes including death or clinical deterioration16,17. These studies pre-dated modern echocardiographic evaluation of MS, and the diagnosis of MS was based on clinical examination by auscultation with or without haemodynamic studies by cardiac catheterisation18. In the contemporary era, relatively little information is known about the predictors of outcome in rheumatic MS, with information mainly provided by retrospective cohort studies. A recent Korean study demonstrated that an elevated PASP is associated with a more rapid progression in PASP which in turn was associated with all-cause mortality and heart failure hospitalisation19. Elevated PASP in MS is a manifestation of the hemodynamic effect of elevated left atrial pressures on the pulmonary circulation. When left uncorrected, it may lead to the development of elevated pulmonary vascular resistance and pulmonary hypertension which has been associated with poorer survival in patients undergoing mitral valve surgery20. LAVI has recently been described as a prognostic factor in rheumatic MS, with two Korean studies demonstrating an association between LAVI and a composite of all-cause death, heart failure admission, MV intervention and stroke21,22. One study, solely focusing on patients with progressive (non-severe) MS, found the severity of MS, LAVI, AF and left ventricular mass index to be associated with composite outcomes; the second in patients with all severities of MS, found LAVI and severity of MS to be significant. Another small Korean study, in patients with mixed MS and MR, found transmitral mean pressure gradient to be solely predictive of a similar composite outcome23. However, a separate case–control study demonstrated that LA volume was not able to predict the development of AF in patients with rheumatic MS24.
Overall, existing studies to date with respect to determinants of outcomes in rheumatic MS are limited and provided conflicting conclusions. Intuitively, worsening severity of MS and increasing age would be expected to negatively affect clinical outcomes, as demonstrated by our findings showing that Yeo’s index and age were independently associated with the composite outcomes. In our study, we did not find an independent association between LAVI and the composite outcome. One explanation could be that our study included relatively older patients who had higher prevalence of comorbidities such as hypertension, diabetes mellitus and vascular disease which are associated with diastolic dysfunction. Diastolic dysfunction can cause an increase in LAVI and in turn confounded any association between LAVI and the severity of MS. In our study, PASP was not independently associated with the composite outcome. The reason for this is unclear. Beyond the studies previously discussed which primarily looked at survival or heart failure hospitalisation outcomes, data regarding the role of PASP in rheumatic MS is scarce19,20.
We did not find an association between AF and the composite outcome probably because more than half of the study patients had AF at baseline. We were unable to include AF in the composite outcome as slightly more than half of the patients had pre-existing AF. In the subgroup analysis of patients without pre-existing AF, we found an independent association between Yeo’s index and new onset AF whereas MVA by planimetry and PHT were not associated with new onset AF in the subgroup analysis.
Limitations
The retrospective study design is the main limitation of this study and limits conclusions regarding the associations we have identified. We were only able to identify associations between Yeo’s index and the composite clinical outcome, but were not able to demonstrate a cause-and-effect relationship between the two due to the retrospective nature of this study. As over half of the patients in our study had pre-existing AF, we did not include new onset AF in the composite outcome, though we found that Yeo’s index was independently associated with new onset AF in subgroup analysis in patients without pre-existing AF. The findings of the subgroup analysis may be limited due to inadequate power from the small subgroup sample size (n = 130) as well as the risk of multiplicity due to multiple comparisons, and should be confirmed by further study.
Conclusion
In patients with rheumatic MS, Yeo’s index was independently associated with a composite outcome of all cause death, heart failure hospitalisation, MV intervention and stroke or TIA which was primarily driven by its association with MV intervention. Subgroup analysis in patients without pre-existing AF showed an independent association between Yeo’s index and new onset AF. We showed that Yeo’s index can be a useful complementary tool to existing measures of MVA for the assessment of rheumatic MS.
Author contributions
RL, TYWL and CMW undertook data collection and statistical analysis. RL and TYWL prepared the manuscript. WK, SPC, KKP, IK, CHS and TCY provided supervision for the project and revised the manuscript. All authors reviewed the manuscript.
Funding
CHS was supported by the National University of Singapore’s Junior Academic Fellowship Scheme as well as the Singapore Ministry of Health National Medical Research Council’s Transition Award (MOH-001368-00).
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to applicable local data protection laws but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the institutional review board, the National Healthcare Group Domain Specific Review Board, reference number 2021/00603. Patient consent was waived by the institutional review board, the National Healthcare Group Domain Specific Review Board, as this was a retrospective cohort study with no potentially identifiable data.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ryan Leow and Tony Yi-Wei Li contributed equally to this work.
References
- 1.Chandrashekhar, Y., Westaby, S. & Narula, J. Mitral stenosis. Lancet374(9697), 1271–1283. 10.1016/S0140-6736(09)60994-6 (2009) (Epub 2009 Sep 9). [DOI] [PubMed] [Google Scholar]
- 2.Iung, B. et al. A prospective survey of patients with valvular heart disease in Europe: The EuroHeart Survey on valvular heart disease. Eur. Heart J.24, 1231–1243 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Otto, C. M., Nishimura, R. A., Bonow, R. O. et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation143(5), e35–e71. 10.1161/CIR.0000000000000932 (2021) (Epub 2020 Dec 17). Erratum in: Circulation. 2021 Feb 2;143(5):e228. Erratum in: Circulation. 2021 Mar 9;143(10):e784. [DOI] [PubMed]
- 4.Boon, N. A. & Bloomfield, P. The medical management of valvar heart disease. Heart87, 395–400 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts, W. C. & Perloff, J. K. Mitral valvular disease a clinicopathologic survey of the conditions causing the mitral valve to function abnormally. Ann. Intern. Med.77, 939–975 (1972). [DOI] [PubMed] [Google Scholar]
- 6.Silbiger, J. J. Advances in rheumatic mitral stenosis: Echocardiographic, pathophysiologic, and hemodynamic considerations. J. Am. Soc. Echocardiogr.34(7), 709–22.e1 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Faletra, F. et al. Measurement of mitral valve area in mitral stenosis: Four echocardiographic methods compared with direct measurement of anatomic orifices. J Am Coll Cardiol.28(5), 1190–1197 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Rifkin, R. D., Harper, K. & Tighe, D. Comparison of proximal isovelocity surface area method with pressure half-time and planimetry in evaluation of mitral stenosis. J. Am. Coll. Cardiol.26(2), 458–465. 10.1016/0735-1097(95)80023-a (1995). [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner, H. et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J. Am. Soc. Echocardiogr.22, 1–23 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Wunderlich, N. C., Beigel, R. & Siegel, R. J. Management of mitral stenosis using 2D and 3D echo-Doppler imaging. JACC Cardiovasc. Imaging6(11), 1191–1205. 10.1016/j.jcmg.2013.07.008 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Sagie, A. et al. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: Valve area and right heart disease. J. Am. Coll. Cardiol.28(2), 472–479 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Leow, R. et al. Yeo’s index: A novel index that combines anatomic and haemodynamic assessment of the severity of mitral stenosis. Int. J. Cardiol.392, 131350. 10.1016/j.ijcard.2023.131350 (2023) (Epub 2023 Sep 7). [DOI] [PubMed] [Google Scholar]
- 13.Seow, S. C., Koh, L. P. & Yeo, T. C. Hemodynamic significance of mitral stenosis: Use of a simple, novel index by 2-dimensional echocardiography. J. Am. Soc. Echocardiogr.19(1), 102–106. 10.1016/j.echo.2005.07.012 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Oktay, A. A. et al. Dimensionless index of the mitral valve for evaluation of degenerative mitral stenosis. Echocardiography37(10), 1533–1542. 10.1111/echo.14847 (2020) (Epub 2020 Sep 7). [DOI] [PubMed] [Google Scholar]
- 15.Shrout, P. E. & Fleiss, J. L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull.86(2), 420–428. 10.1037/0033-2909.86.2.420 (1979). [DOI] [PubMed] [Google Scholar]
- 16.Rowe, J. C., Bland, E. F., Sprague, H. B. & White, P. D. The course of mitral stenosis without surgery: Ten and twenty year perspectives. Ann. Intern. Med.52, 741–749 (1960). [DOI] [PubMed] [Google Scholar]
- 17.Olesen, K. H. The natural history of 271 patients with mitral stenosis under medical treatment. Br. Heart J.24(3), 349–357. 10.1136/hrt.24.3.349 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selzer, A. & Cohn, K. E. Natural history of mitral stenosis: A review. Circulation45(4), 878–890. 10.1161/01.cir.45.4.878 (1972). [DOI] [PubMed] [Google Scholar]
- 19.Ko, K. Y. et al. Identification of distinct subgroups in moderately severe rheumatic mitral stenosis using data-driven phenotyping of longitudinal hemodynamic progression. J. Am. Heart Assoc.11(15), e026375. 10.1161/JAHA.121.026375 (2022) (Epub 2022 Jul 29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, B. et al. The impact of concomitant pulmonary hypertension on early and late outcomes following surgery for mitral stenosis. J. Thorac. Cardiovasc. Surg.152(2), 394–400.e1. 10.1016/j.jtcvs.2016.02.038 (2016) (Epub 2016 Feb 24). Erratum in: J Thorac Cardiovasc Surg. 2016 Nov;152(5):1465. [DOI] [PubMed]
- 21.Cho, I. J. et al. Prognostic implications of the left atrial volume index in patients with progressive mitral stenosis. J. Cardiovasc. Imaging27(2), 122–133. 10.4250/jcvi.2019.27.e20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho, I. J., Jeong, H. & Chang, H. J. Prognostic value of left atrial volume index in patients with rheumatic mitral stenosis. Clin. Cardiol.44(3), 364–370. 10.1002/clc.23544 (2021) (Epub 2021 Jan 6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho, I. J., Lee, S. E., Jeong, H. & Chang, H. J. Determinants of clinical outcomes in patients with mixed mitral valve disease. Echocardiography37(8), 1164–1170. 10.1111/echo.14673 (2020) (Epub 2020 Jul 12). [DOI] [PubMed] [Google Scholar]
- 24.Ancona, R. et al. Two-dimensional atrial systolic strain imaging predicts atrial fibrillation at 4-year follow-up in asymptomatic rheumatic mitral stenosis. J. Am. Soc. Echocardiogr.26(3), 270–277. 10.1016/j.echo.2012.11.016 (2013) (Epub 2012 Dec 20). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to applicable local data protection laws but are available from the corresponding author on reasonable request.