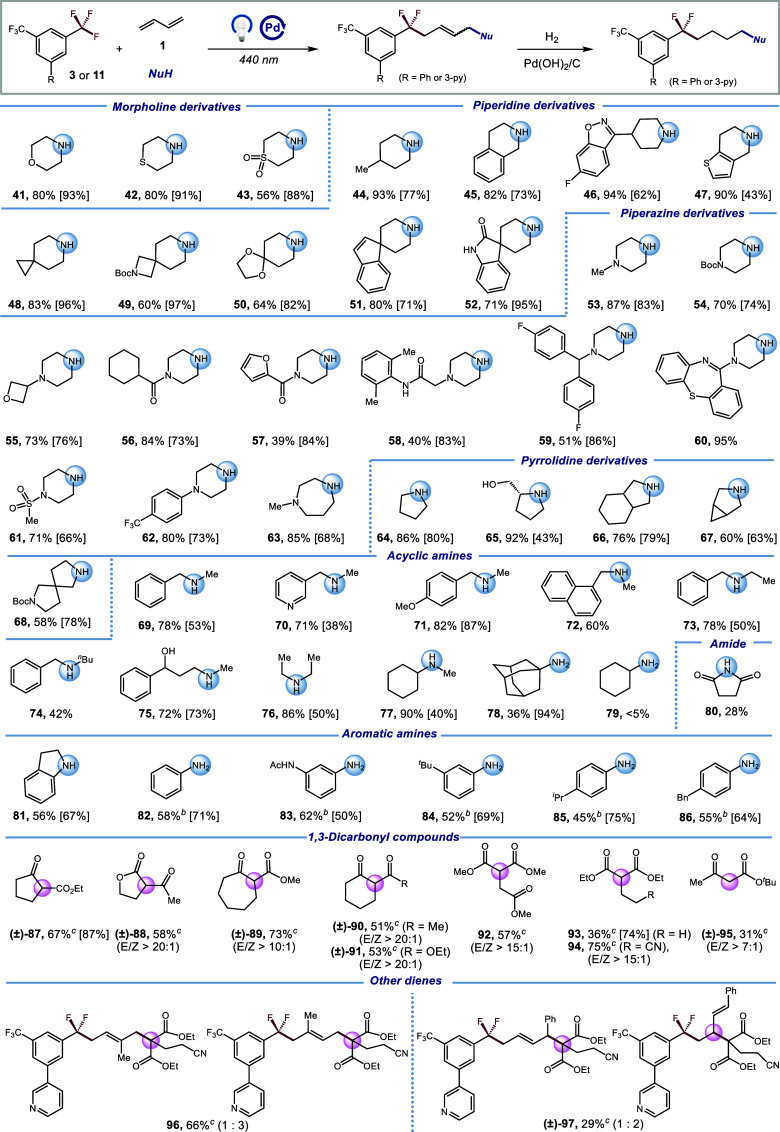
Scheme 2. Scope of Nucleophiles.
Reaction conditions: 3 (0.9 mmol), 1 (0.6 mmol), amine (0.3 mmol), Pd(PPh3)4 (1.5 mol%), XantPhos (8 mol%), [(o-OMe)Ph]2PPh (8 mol%), LiOH (0.3–0.9 mmol, when amine hydrochloride was used, 1.0 equiv. of hydrochloride consumed additional 1.0 equiv. of LiOH), THF (0.1 M), λmax = 440 nm Kessil (40 W), N2, RT–40 °C, 12 h. Hydrogenation yield is shown in square brackets. See Supporting Information for hydrogenation procedures.
w/o [(o-OMe)Ph]2PPh, with Mg(OTf)2 (20 mol%).
11 (0.9 mmol), 1 (0.6 mmol), 1,3-dicarbonyl compound (0.3 mmol), Pd(PPh3)4 (1.5 mol%), XantPhos (8 mol%), [(o-OMe)Ph]2PPh (8 mol%), LiOH (0.3 mmol), THF (0.1 M), λmax = 440 nm Kessil (40 W), N2, RT– 40 °C, 12 h. Hydrogenation yield is shown in square brackets. See Supporting Information for hydrogenation procedures.