Abstract
Background
The erythroblast transformation-specific related gene (ERG) is expressed in hematopoietic stem and progenitor cells and endothelial cells. This study aimed to investigate if ERG rs2836411 is a novel genetic locus associated with anemia and aortic dissection (AD).
Method
A case-control trial was conducted to evaluate the association between ERG rs2836411 polymorphism, anemia, and AD risk. The ERG rs2836411 polymorphism was analyzed using Sanger dideoxy chain termination sequencing on genomic DNA extracted from whole blood. Serum erythropoietin (EPO) and interleukin-6 (IL-6) concentrations were measured by enzyme-linked immunosorbent assay (ELISA).
Results
119 preoperative AD patients and 119 healthy controls were enrolled, with age and sex matched. Anemia was found independently associated with AD presence (Odds ratio (OR) 20.82, p < 0.001). Remarkably, T carriers (CT + TT) of ERG rs2836411 were associated with anemia in AD patients (OR 2.81, p = 0.013) but not in controls. After adjusting for conventional risk factors including age, sex, smoking and hypertension status, T carriers (CT + TT) were independently associated with AD presence (OR 2.20, p = 0.015), but were not associated if anemia was further adjusted. While EPO concentration was higher in AD patients and was associated with AD presence (OR 1.09, p = 0.006), no difference in EPO levels was observed between the ERG genotypes of AD patients.
Conclusions
T carriers (CT + TT) of ERG rs2836411 are independently associated with anemia and AD presence. The association between ERG rs2836411 polymorphism and susceptibility to AD may be mediated by anemia. Further studies are warranted to validate whether this association is causal.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04345-5.
Keywords: Aortic dissection, ERG, Single nucleotide polymorphism, Anemia, Erythropoietin
Introduction
Aortic dissection (AD) is a life-threatening disorder worldwide, which may be asymptomatic before disease onset, but will eventually require emergency surgery [1]. Due to the extremely high mortality rate, prevention and early diagnosis have become critical measures for improving the survival probability of AD patients. Predictive markers or indicators that may accurately identify the group at high risk of AD should be screened since they have significant clinical importance. Low level of hemoglobin (Hb) has been shown to be a predictive factor for poor cardiovascular outcomes in previous studies [2]. We and others recently demonstrated that preoperative anemia is independently associated with the short- and long-term worse outcomes in AD patients following thoracic endovascular aortic repair (TEVAR) [3, 4]. However, it remains unexplored if preoperative anemia is associated with the susceptibility to AD.
The erythroblast transformation-specific related gene (ERG) is located in chromosomal region 21q22.2 and contains a total of 17 exons and 1085 single nucleotide polymorphisms (SNPs) with a minor allele frequency ≥ 0.05 throughout its entire sequence. Of note, rs2836411 is situated within a super-enhancer region that starts from upstream of P3 to downstream of exon 6, with high levels of histone 3 lysine 27 acetylation (H3K27ac) and histone 3 lysine 4 monomethylation (H3K4me1) [5]. Recently, rs2836411 has been discovered as a novel SNP and the only one SNP located within the ERG gene, linked to abdominal aortic aneurysm (AAA) by genome-wide association studies [6]. Compared to the protective C allele, the risk T allele demonstrated an inability to form a sub-domain structure with the super-enhancer, potentially leading to diminished enhancer activity and, consequently downregulated ERG gene expression [5]. Given the important role of ERG in mediating vascular development and inflammation in atherosclerosis [7, 8], and the fact that rs2836411 was not in high linkage disequilibrium with any other SNP [5], rs2836411 was suggested to be a potential mediator of AAA formation by reducing endothelial-derived ERG expression. In addition, ERG is also critical for hematopoietic cell differentiation and definitive hematopoiesis [7, 9]. ERG has been also shown to modulate Notch signaling and controls the balance between Notch ligands [10], which regulates not only hematopoietic precursors and differentiation, but also adult erythropoiesis, particularly in early erythroid precursors [11]. Given that definitive hematopoiesis governs the development of erythroid cells, a decrease in ERG expression may directly impair red blood cell production. However, whether the risk allele T of rs2846411 is associated with the risk of anemia by affecting hematopoietic ERG expression remains unexplored. To date, while rs2836411 is the only SNP with evidence supporting its regulatory role in ERG expression and its association with AAA, no studies have investigated the association between ERG rs2836411 polymorphism and anemia, and its potential role in mediating the susceptibility to AD.
Erythropoietin (EPO) is tightly regulated by oxygen levels and hemoglobin concentrations, and significantly increases in response to hypoxia or hemoglobin decline [12]. Several large clinical trials have found that high EPO levels are associated with an increased risk of heart failure and adverse events [13, 14]. Previous report has documented a rare AAA patient receiving both chronic dialysis and periodic recombinant human EPO replacement therapy [15]. Recent basic research has also demonstrated a link between EPO and AAA pathogenesis [16]. As anemia is prevalent among AD patients, it is theoretically expected that AD patients may exhibit elevated EPO levels. Thus, it is essential to explore whether the plasma EPO levels in AD patients differ from those in controls, and whether the relationship between ERG rs2836411 polymorphism, anemia and AD is associated with EPO.
Therefore, in the current study, we aimed to address the association between ERG rs2836411 polymorphism, anemia, and susceptibility to AD. Meanwhile, we tested the potential role of EPO in this relationship.
Methods
Study population
This study was a single-center, case-control study conducted at the Second Affiliated Hospital (Xinqiao Hospital) of Army Medical University in Chongqing, China. Briefly, 238 participants (119 AD patients and 119 age- and sex-matched controls) were consecutively enrolled from March 2022 to August 2022. All the AD patients were diagnosed through computed tomography-angiography (CTA). The AD patients were triaged by the Emergency Department and had autonomy in deciding whether to undergo further treatment, regardless of the severity of their condition. The patients with following diseases were excluded: (1) Marfan’s syndrome, (2) Severe heart failure (HF) (New York Heart Association (NYHA) class III-IV), (3) Chronic renal failure, (4) Polycythemia, (5) Malignant tumors. The control subjects were recruited from the same hospital without aortic diseases and followed the same excluded criteria as AD patients. All the participants were informed with the research contents and signed informed consent forms. This study was approved by the ethics committee of Second Affiliated Hospital of Army Medical University (https://www.chictr.org.cn, identification code: ChiCTR2200059302) and carried on according to the guidelines of the Declaration of Helsinki.
Clinical data and laboratory testing
The demographics and medical history of the study subjects were collected from medical records. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Smoking was defined as smoking more than one cigarette/day in the last 6 months. Anemia was defined as a Hb level < 120 g/L for men and < 110 g/L for women according to Chinese criteria [17]. Based on morphological classification, anemia was categorized as microcytic or normocytic [18]. The plasma samples were obtained simultaneously with the initial blood draw required for laboratory tests from the study subjects and stored at -80 °C until used. Plasma EPO (Quantikine, R&D Systems, Minneapolis, USA) and interleukin-6 (IL-6) (Thermo Fisher Scientific, Waltham, MA, USA) concentration was measured by enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. Other blood indices, including serum creatinine, cystatin-C, and red blood cell (RBC) count were measured by the clinical laboratory of the hospital.
Genotyping
Genomic DNA was extracted from peripheral blood using the QIAmp Blood Kit (QIAGEN, Hilden, Germany). Single nucleotide polymorphisms and genotypes of ERG rs2836411 were analyzed using Sanger dideoxy chain termination method (ABI 3730XL DNA Analyzer, Applied Biosystems, CA, USA). DNA was amplified in PCR and subjected to chain termination using dideoxynucleotides (ddNTPs). The resulting fragments were then separated by capillary electrophoresis, generating a fluorescence spectrum that reflects the nucleotide sequence of the amplified DNA. Primers were designed on the conserved sequences flanking the SNP loci, utilizing the design tools available on the National Center for Biotechnology Information website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi) (Table S1). PCR amplification, PCR purification and DNA recovery were implemented according to the manufacturer’s protocol. Allele detection was performed by analyzing peak identification in fluorescence chromatograms using the Chromas software (ver. 2.6.6, Technelysium Pty Ltd, South Brisbane, AUS). Homozygous alleles were identified by a single, distinct peak at the variant position, whereas heterozygous alleles were indicated by two overlapping peaks of approximately equal height, corresponding to both alleles. Genotyping was performed using a blinded method and 10% of the samples were reanalyzed to avoid potential genotyping errors.
Statistical analysis
Continuous variables were expressed as means ± SD or median (1st quartile − 3rd quartile). Intergroup comparisons were performed using two-tailed unpaired Student’s t test (normally distributed data) or Mann-Whitney U test (non-normally distributed data). Categorical variables were expressed as n (%) and intergroup comparisons were performed using chi-square (χ2) test. Hardy–Weinberg equilibrium (HWE) was analyzed to verify the balance of allele frequencies and genotype distributions between groups. Correlation between T allele of ERG rs2836411 and the variables were analyzed by spearman correlation analysis. Univariate and multivariate binary logistic regression analyses were performed to analyze the association of anemia and AD. Statistical analyses were performed using SPSS software (ver. 24.0, SPSS Inc., Chicago, IL, USA) and SNPStats (https://www.snpstats.net/) [19]. The difference was considered statistically significant at p value < 0.05.
Results
Clinical data of study population
A total of 119 AD patients and 119 controls with age- and sex-matched were enrolled in the current study (Figure S1). Clinical characteristics of the study population were presented in Table 1. Compared with controls, AD patients had a significantly higher incidence of hypertension (23.5% vs. 68.9%, p < 0.001). The blood indices, including Hb, hematocrit (HCT), RBC count, platelet (PLT) count and mean corpuscular hemoglobin concentration (MCHC) were significantly lower in AD patients compared with those in controls. The levels of IL-6, C-reactive protein (CRP), white blood cell and neutrophilic granulocyte were significantly higher in AD patients compared with those in controls. Of note, AD patients exhibited significantly higher plasma levels of EPO compared with those of control subjects (10.4 vs. 15.7, p < 0.001).
Table 1.
Baseline characteristics of AD patients and controls
| Characteristics | Controls (n = 119) | AD patients (n = 119) | p-value |
|---|---|---|---|
| Age (years) | 56.9 ± 12.6 | 57.1 ± 12.8 | 0.862 |
| Males (n, %) | 100 (84.0%) | 100 (84.0%) | 1.000 |
| Stanford Type | |||
| A | N/A | 64 (53.8%) | N/A |
| B | N/A | 55 (46.2%) | N/A |
| Smoking (n, %) | 55 (46.2%) | 58 (48.7%) | 0.823 |
| Hypertension (n, %) | 28 (23.5%) | 82 (68.9%) | < 0.001 |
| Anemia (n, %) | 3 (2.5%) | 33 (27.7%) | < 0.001 |
| Normocytic anemia | 3 (100%) | 26 (78.8%) | N/A |
| Microcytic anemia | 0 (0%) | 7 (21.2%) | |
| Interleukin-6 (pg/mL) | 5.0 (3.1–13.7) | 28.9 (18.1–73.4) | < 0.001 |
| C-reactive protein (mg/L) | 5.6 (5.0-12.5) | 14.6 (5.0-108.2 | 0.006 |
| WBC (×109/L) | 6.0 (5.2–7.4) | 10.7 (7.8–14.0) | < 0.001 |
| NEUT (×109/L) | 3.5 (3.0-4.8) | 8.7 (6.0–12.0) | < 0.001 |
| Hb (g/L) | 147.9 ± 14.3 | 127.6 ± 19.3 | < 0.001 |
| RBC (×109/L) | 4.8 ± 0.5 | 4.2 ± 0.6 | < 0.001 |
| HCT (%) | 44.2% ± 3.9% | 38.7% ± 5.3% | < 0.001 |
| MCV (fL) | 91.7 ± 5.3 | 91.5 ± 6.6 | 0.773 |
| MCH (pg) | 30.7 ± 2.0 | 30.4 ± 2.6 | 0.294 |
| MCHC (g/L) | 334.7 ± 7.6 | 331.9 ± 9.4 | 0.014 |
| PLT (×109/L) | 199.9 ± 53.0 | 164.5 ± 65.9 | < 0.001 |
| EPO (U/L) | 10.4 (7.5–13.4) | 15.7 (9.1–25.7) | < 0.001 |
Data are expressed as n (%) or mean ± standard deviation (SD) or median (1st quartile − 3rd quartile). AD: aortic dissection; BMI: body mass index; N/A: not applicable; WBC: white blood cell; NEUT: neutrophilic granulocyte; Hb: hemoglobin; RBC: red blood cell; HCT: hematocrit; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; PLT: platelet; EPO: erythropoietin
Association of anemia with aortic dissection
As shown in Table 1, the frequency of anemia was significantly higher in AD patients compared with that in controls (2.5% vs. 27.7%, p < 0.001). Normocytic anemia was the predominant type in AD patients. The baseline characteristics exhibited no significant variation between AD patients with normocytic anemia and microcytic anemia (Table S2). We further analyzed the association between anemia and the presence of AD. After adjusting for classic AD risk factors, including age, sex, smoking and hypertension status, anemia was associated with AD (OR 20.82, 95% CI = 5.38–80.51, p < 0.001). To further explore the potential mechanisms underlying the association between anemia and AD, a subgroup analysis was conducted. Compared with AD patients without anemia, patients with anemia showed higher levels of IL-6 (Table S3). Collectively, these data suggest that anemia is associated with AD and inflammation.
Allele frequency and genotype distribution of ERG rs2836411 in aortic dissection patients and controls
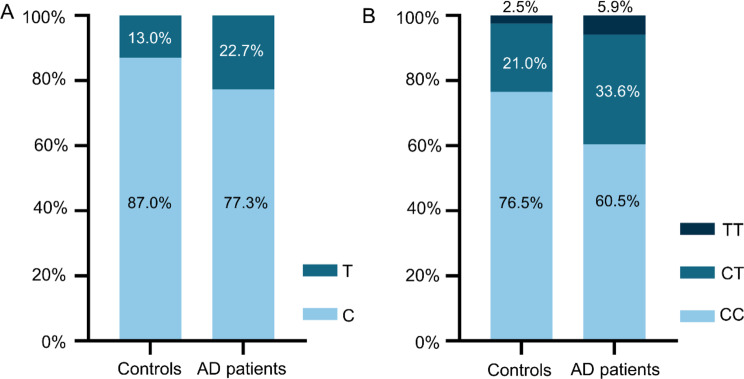
The characteristics of allele frequencies and genotype distributions of ERG rs2836411 in controls and AD patients were shown in Fig. 1. The allele frequencies and genotypes of ERG rs2836411 in both groups followed the Hardy-Weinberg equilibrium (p >0.05). In controls, the C allele accounted for 87% and the T allele accounted for 13%. In AD patients, the C allele accounted for 77.3% and the T allele accounted for 22.7%. The T allele of ERG rs2836411 represents the minor allele for this SNP, and the T allele frequency in AD patients was significantly higher compared with that in controls (p = 0.006). The frequencies of genotypes CC, CT and TT were 76.5%, 21%, and 2.5% respectively in controls, and were 60.5%, 33.6% and 5.9% respectively in AD patients. Furthermore, the differences of genotype distributions between cases and controls were compared in 3 models. Specifically, in the codominant model (CC vs. CT vs. TT), genotype distributions were significantly different between cases and controls (p = 0.026). In the dominant model (CC vs. CT + TT), the frequencies of T carriers were 23.5% in controls and 39.5% in AD patients, with a statistically significant difference (p = 0.008). In the recessive model (CC + CT vs. TT), the frequencies of TT genotype were 2.5% and 5.9% in controls and AD patients respectively, however, the difference was not significant due to the small number of TT genotype in both groups. Therefore, we focused on analyzing the differences between CC and T carriers (CT + TT) in the dominant model. Overall, these data suggest that T allele and T carriers of ERG rs2836411 are significantly more frequent in AD patients compared with those in controls.
Fig. 1.
Allele frequencies and genotype distributions of ERG rs2836411 in controls and AD patients. A. Allele frequencies of ERG rs2836411 in controls and AD patients. B. Genotype distributions of ERG rs2836411 in controls and AD patients
Association of ERG rs2836411 polymorphism with anemia
We investigated the correlation between T carriers of ERG rs2836411 and the variables. As shown in Table 2, T carriers of ERG rs2836411 were positively associated with anemia (p = 0.012) in AD groups, particularly normocytic anemia (p = 0.009), and negatively associated with RBC counts (p = 0.018), but not associated with CRP and IL-6 levels. No significant difference in ERG gene frequency was discerned among the AD patients with normocytic anemia and microcytic anemia (Table S4). To further identify if ERG rs2836411 gene polymorphism was associated with anemia, univariate logistic regression analyses were performed (Table 3). Specifically, in the codominant model, CT and TT genotypes of ERG rs2836411 were significantly associated with anemia in the group of AD patients. Similarly, in the dominant model, the T carriers (CT + TT) were significantly associated with anemia in AD patients (CT + TT vs. CC, OR 2.81, 95% CI = 1.23–6.41, p = 0.013), but not in controls. Although the TT genotype became significantly associated with anemia in controls but not in AD patients in the recessive model, the small number of TT genotype in both groups limited the interpretation of this data. Overall, these results supported the hypothesis that T allele and T carriers of ERG rs2836411 were associated with anemia in AD patients but not in controls.
Table 2.
Correlation between T carriers of ERG rs2836411 and selected covariates
| Variable | Controls (n = 119) | AD patients (n = 119) | |||
|---|---|---|---|---|---|
| r | p-value | r | p-value | ||
| Age (years) | 0.007 | 0.938 | 0.172 | 0.061 | |
| BMI (kg/m2) | -0.020 | 0.852 | 0.049 | 0.619 | |
| Stanford Type | N/A | N/A | -0.113 | 0.221 | |
| Hypertension (n, %) | 0.066 | 0.476 | -0.051 | 0.578 | |
| Anemia (n, %) | 0.037 | 0.688 | 0.229 | 0.012 | |
| Normocytic anemia | 0.037 | 0.688 | 0.238 | 0.009 | |
| Microcytic anemia | N/A | N/A | 0.017 | 0.853 | |
| Interleukin-6 (pg/mL) | 0.314 | 0.274 | -0.017 | 0.868 | |
| C-reactive protein (mg/L) | 0.127 | 0.604 | 0.022 | 0.818 | |
| WBC (×109/L) | -0.085 | 0.356 | 0.066 | 0.475 | |
| NEUT (×109/L) | -0.058 | 0.533 | 0.059 | 0.525 | |
| Hb (g/L) | -0.082 | 0.374 | -0.180 | 0.050 | |
| RBC (×109/L) | -0.018 | 0.240 | -0.217 | 0.018 | |
| HCT (%) | -0.078 | 0.398 | -0.137 | 0.136 | |
| MCV | -0.025 | 0.786 | 0.080 | 0.396 | |
| MCH | 0.003 | 0.978 | 0.070 | 0.458 | |
| MCHC | 0.102 | 0.270 | 0.037 | 0.693 | |
| PLT (×109/L) | -0.053 | 0.564 | -0.172 | 0.061 | |
| EPO (U/L) | 0.055 | 0.554 | 0.091 | 0.327 | |
AD: aortic dissection; N/A: not applicable; BMI: body mass index; WBC: white blood cell; NEUT: neutrophilic granulocyte; Hb: hemoglobin; RBC: red blood cell; HCT: hematocrit; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; PLT: platelet; EPO: erythropoietin
Table 3.
Association between ERG rs2836411 polymorphisms and anemia
| Model | Controls | AD patients | |||||
|---|---|---|---|---|---|---|---|
| Genotype frequency | OR (95% CI) | p-value | Genotype frequency | OR (95% CI) | p-value | ||
| Codominant | CC (76.5%) | 1 | CC (60.5%) | 1 | 0.029 | ||
| CT (21.0%) | - | - | CT (33.6%) | 2.49 (1.05–5.91) | |||
| TT (2.5%) | 22.25 (1.38–358.50) | 0.029 | TT (5.9%) | 5.52 (1.11–27.54) | |||
| Dominant | CC (76.5%) | 1 | CC (60.5%) | 1 | 0.013 | ||
| CT + TT (23.5%) | 1.65 (0.14–18.89) | 0.688 | CT + TT (39.5%) | 2.81 (1.23–6.41) | |||
| Recessive | CC + CT (97.5%) | 1 | CC + CT (94.1%) | 1 | 0.092 | ||
| TT (2.5%) | 28.50 (1.77-458.39) | 0.046 | TT (5.9%) | 3.82 (0.81–18.08) | |||
AD: aortic dissection; OR: odds ratio; 95% CI: 95% confidence interval
Association of ERG rs2836411 polymorphism with the susceptibility to aortic dissection
To investigate the association between ERG rs2836411 polymorphism and AD, both univariate and multivariate logistic regression analyses were performed in Fig. 2. In the univariate logistic regression analysis, T carriers (CT + TT) were significantly associated with AD presence (CT + TT vs. CC, OR 2.12, 95%CI = 1.21–3.72, p = 0.009) in dominant model. In the multivariate regression analyses, after adjusting for conventional AD risk factors including age, sex, smoking and hypertension status, T carriers (CT + TT) of ERG rs2836411 gene polymorphism remained independently associated with AD presence (CT + TT vs. CC, OR 2.20, 95%CI = 1.17–4.14, p = 0.015) in dominant model. Notably, the association between ERG rs2836411 polymorphism and AD remained significant when further adjusted for EPO but became insignificant when further adjusted for anemia in this multivariate regression analysis. Collectively, these results suggest that T allele and T carriers of ERG rs2836411 are significantly associated with anemia and the susceptibility to AD.
Fig. 2.
Forest plot of univariate and multivariate analyses of correlation between ERG rs2836411 polymorphism and AD. A. Multivariate binary logistic regression analyses were adjusted for age, sex, smoking, and hypertension status. B. Multivariate binary logistic regression analyses were adjusted for age, sex, smoking, hypertension, and erythropoietin. C. Multivariate binary logistic regression analyses were adjusted for age, sex, smoking, hypertension, and anemia status. OR: odds ratio; 95% CI: 95% confidence interval
Association of EPO with anemia and ERG rs2836411 Polymorphism
To further explore the association between EPO with anemia and AD, we compared the plasma EPO levels between non-anemia and anemia groups within the AD patients. EPO levels were higher in anemia group (14.2 vs. 22.2, p = 0.030) and Spearman correlation analysis (r=-0.339, p < 0.001) indicated significant correlation between EPO and Hb (Figure S3A, S3B). EPO levels were independently associated with AD (Figure S3D). Furthermore, we compared the levels of EPO in CC genotype and T carriers (CT + TT) groups of AD patients. However, there was no difference in plasma EPO levels between the CC genotype and T carriers (CT + TT) groups (Figure S3C). Overall, the association between ERG rs2836411 polymorphism and susceptibility to AD may not be mediated through EPO in the current small sample size.
Discussion
In the present study, we report for the first time that preoperative anemia is associated with AD presence and that preoperative anemia in AD patients is associated with a single nucleotide mutation at the ERG rs2836411 locus. Furthermore, we found that the risk allele T and T carriers of ERG rs2836411 were independently associated with susceptibility to AD. Of note, this correlation might be primarily attributed to the intrinsic association between ERG rs2836411 and anemia.
While a limited number of studies have explored the potential association between anemia and AD, these investigations have largely ignored the possible role anemia may play in the onset of AD. Recently, anemia has been recognized not merely as a reduction in hemoglobin levels impairing oxygen transport, but also as a condition with direct detrimental effects on the cardiovascular system. Honda et al. demonstrated ongoing myocardial injury in the context of anemia by measuring heart-type fatty acid-binding protein (H-FABP) levels. Anemia was a predictor of cardiovascular mortality in the healthy population [20]. Furthermore, the most recent Mendelian randomization study has addressed the bidirectional causality between anemia and HF, as well as significant associations between genetic susceptibility to CAD with anemia [21], highlighting the underestimated role of anemia in the pathogenesis of cardiovascular diseases and the mediating role of genetic factors. In the context of AD, our previous study mainly explored the prognostic value of anemia for AD, without considering the potential possibility of a direct impact of anemia on aortic integrity [3]. Although the sequential relationship between preoperative anemia and the onset of AD remained unclear, the current mainstream view believed that anemia was a complication of AD, and AD might cause anemia in a variety of ways, including: (1) blood loss by partial rupture of the dissection; (2) necrosis, rupture, and hemorrhage of gastrointestinal blood vessels due to inadequate perfusion; and (3) aortic wall hematoma [22]. However, no research has yet explored if anemia is associated with susceptibility to AD.
Previous studies have demonstrated that anemia can lead to tissue and organ hypoxia, and it is well known that hypoxia can upregulate inflammatory responses through multiple pathways [23]. Hypoxia directly damages the endothelial and epithelial barrier, enhancing inflammatory cell infiltration and upregulating expression of pro-inflammatory cytokines and chemokines in the circulation [24, 25]. Consistently, we found in the current study that the levels of inflammation in AD and the incidence of anemia were significantly higher compared to the control group. More importantly, compared to AD patients without anemia, AD patients with anemia showed higher levels of IL-6, which indicated that AD patients with anemia demonstrated a more pronounced inflammatory response. Inflammatory responses can induce the transformation of vascular smooth muscle cells (VSMCs), which are an essential constituent of the arterial wall, from a dormant contractile phenotype to an active, highly migratory, and proliferative synthetic phenotype [26]. This transformation triggers significant degradation of the extracellular matrix (ECM), leading to the detachment of VSMCs from the ECM, ultimately causing the migration and apoptosis of VSMCs. The subsequent reduction in both VSMCs and ECM weakens the aortic wall’s structure, enhancing its predisposition towards dilation and setting in motion the genesis and advancement of AD. Given that inflammation is a critical mechanism involved in the pathogenesis of AD [27], anemia-related endothelial damage and inflammation may contribute to the occurrence of aortic dissection. Furthermore, the hemodynamic disturbances caused by anemia may also increase the risk of developing AD. As concluded by previous studies, AD occurs more frequently in the aorta exposed to pulsed blood flow [28]. Aortic intimal tear occurs due to normal and shear stresses in the aortic wall, which are initially caused by an increase in average and maximum aortic pressure as well as anisotropy of the aorta. The increase in heart rate and pulse pressure can exacerbate the hemodynamic stress on the aortic wall, promoting AD progression [29]. During anemia, the hemodynamic changes of increased systemic arterial dilation and decreased systemic vascular resistance ultimately lead to an increase in stroke output [30]. In addition, activation of the sympathetic nervous system can cause an increase in heart rate, while reduced blood viscosity and increased venous reflux may contribute to a rise in preload [31]. Altogether, the increases in preload, heart rate and stroke output may exacerbate the blood flow pulses and maximum aortic pressure, implying a potential mechanism by which anemia may contribute to the susceptibility to AD. There was no observed difference in both the ERG gene frequency and the baseline data among the two types of anemic AD patients. Therefore, we included the anemia status as one factor and tested the hypothesis that anemia is associated with AD presence in the current cohort. Anemia was found to be independently associated with the susceptibility to AD in multivariate models that adjusted for conventional risk factors for AD, suggesting a potentially important role of anemia in the pathogenesis of AD. Thus, investigating the causes of preoperative anemia in AD patients is of great significance for understanding the etiology of AD.
The critical pathological progression in AD involves a reduction in the structural integrity of the aortic wall. The genetic variations that contribute to this instability can be categorized into two main mechanisms. One directly affects key structural proteins during aortic development, leading to congenital defects, and the other indirectly weakens aortic stability by modulating inflammatory pathways. Most previously reported pathogenic genes have only demonstrated one potential mechanism for weakening aortic stability. Ling et al. [32] identified that SNPs located in fibrillin-1 (FBN1) and transforming growth factor-β (TGF-β) were associated with an increased risk of AD. Their findings suggested that mutations caused pathogenic changes in the extracellular matrix by reducing levels of functional microfibrils and interfering with TGF-β signaling, both of which were crucial for maintaining aortic wall integrity and ultimately leading to the development of AD. Jiang et al. [33]reported the association between SNPs in interleukin-1 receptor 1 and AD, indicating that the inflammatory pathways might play a critical role in undermining aortic wall integrity. The above two studies share a common feature, that is, the impact of the genes studied on AD is only discussed through direct or indirect mechanisms.
Among the novel loci that were recently discovered to be associated with AAA, rs2846411 attracted our attention [6]. It mainly interacts within super-enhancer of the ERG gene, which is primarily expressed in vascular endothelial cells and HSPCs [7, 34], and is critical for maintaining vascular development and definitive hematopoiesis [7, 9]. Of note, the risk allele T of rs2846411 was found to significantly downregulate ERG expression by affecting its enhancer activity [5]. Decreased ERG expression is associated with impaired endothelial integrity and hematopoiesis, therefore, we speculated that if the ERG rs2846411 polymorphism might be related to the cause of anemia in AD patients by reducing the expression of ERG.
As mentioned above, ERG is involved in multiple processes of vascular development, including endothelial cell differentiation, reprogramming, angiogenesis, inflammation in the endothelium [7]. Impairment of endothelial integrity due to loss of ERG expression may result in the formation of a proximal dissection rupture. Besides, inflammatory agents can induce a reduction in ERG expression, which in turn elevates vascular permeability and facilitates the accumulation of inflammatory cells, resulting in vascular dysfunction [35]. Notably, knockdown of Erg increases endothelial cell permeability, which exacerbates the infiltration of pro-inflammatory immune cells and thereby promotes inflammatory response [36], Thus, a primary reduction in ERG expression might establish a loop with inflammatory agents, together contributing to the disruption of vascular equilibrium. Moreover, it is widely recognized that anemia is a prevalent condition among patients with chronic inflammatory diseases [37]. Chronic inflammation-induced iron restriction, suppressed erythropoietic activity, and decreased erythrocyte survival are the main mechanisms of inflammatory anemia [38]. As studies found that aortas of AD were in a condition of persistent inflammation during disease progression [27], thus we speculated that inflammation and anemia could be mutually causal, promoting the progression of AD. Overall, deficiency of endothelial-derived ERG expression may be associated with increased risk of endothelial vulnerability, endothelial inflammation, and anemia, ultimately promoting the formation of AD.
The effect of ERG in the hematopoietic system is to maintain definitive hematopoiesis and the self-renewal of hematopoietic stem cells (HSCs) [9, 34]. Homozygous deficiency of Erg can lead to fatal hematopoietic failure in mice. Although mice with heterozygous Erg deficiency can survive, their marrow produces fewer HSPCs and displays a significant inability to compete with normal marrow for hematopoietic reconstitution [9]. Of note, ERG is necessary for the directional differentiation of megakaryocyte erythroid progenitor cells (MEPs) towards the megakaryocyte lineage and the erythrocyte lineage [39]. Overexpression of Erg results in massive proliferation of immature erythrocytes and megakaryocytes, leading to the development of erythro-megakaryocytic leukemia [40]. Conversely, deficiency of Erg may be related to the insufficient differentiation of MEP into mature erythrocytes and megakaryocytes and their insufficient self-proliferation. Moreover, ERG has been identified as a new biomarker of human embryonic stem cell (hESC)-derived hemogenic endothelium (HE) and hematopoietic progenitor (HP) cells. Compared to non-HE, ERG significantly increased HE-related genes in HE and HP [41], suggesting that a decrease in ERG expression may hinder the endothelial-to-hematopoietic transition, and when the body’s demands for hematopoiesis increase, such as chronic inflammation, it ultimately leads to insufficient hematopoiesis.
Consistent with the above assumptions, we found that the frequencies of risk allele T and T carriers of ERG rs2836411 were significantly higher in AD patients compared to those in controls. Among AD patients, those with CT or TT genotypes were significantly associated with anemia compared to those with CC genotypes. Of note, this association between ERG rs2836411 polymorphism and anemia only applied to AD patients but not to controls in the current cohort, highlighting the inherent association between ERG rs2836411 polymorphism and anemia in the context of AD. We further explored if ERG rs2836411 polymorphism is associated with the susceptibility to AD. Indeed, we found that CT and TT genotypes were independently associated with the susceptibility to AD in the multivariate models after adjusting for conventional risk factors for AD, notably, this association became insignificant if further adjusted for anemia.
It is worth noting that a recent study demonstrated a causal link between high serum EPO levels and the progression of AAA [16]. As EPO is primarily produced in response to anemia, we examined the plasma EPO levels in the study population to explore whether the correlation between ERG rs2836411, anemia, and the susceptibility to AD is mediated by EPO. As we found, plasma EPO levels were significantly higher in AD patients compared to those in controls within the current cohort. To further understand if the elevated EPO was secondary to anemia in the context of AD, we conducted additional investigations. In AD patients, EPO levels were higher in the anemia group compared to the non-anemia group and no association was found between EPO and the different anemia types. In addition, Spearman correlation analysis indicated negative correlation between EPO and Hb. These results indicated that EPO might be involved in the development of anemia-associated AD. Although the T carriers did not show elevated levels of EPO under dominant model in the current small cohort, the association between ERG rs2836411 polymorphism, anemia and EPO levels in the context of AD deserved to be extensively investigated in future large-scale cohorts. Occasional outliers of EPO observed in AD without anemia might suggest the potential role of other factors, such as the consequence of compensatorily increased levels of EPO due to decrease in biological activity under inflammation. The reality might be significantly more complex than the straightforward correlations suggested by the data. As demonstrated by Zhang et al. [16], EPO can promote the formation of AAA through enhancement of angiogenesis, inflammation, collagen degradation, and VSMCs apoptosis via EPO/EPOR signaling pathway. Similar pathological changes have been observed in AD tissue [42]. Therefore, it is conceivable that EPO may contribute to the occurrence and progression of AD through similar or overlapping mechanisms found in AAA.
In summary, the present study has demonstrated that anemia is enriched in AD patients and it is associated with susceptibility to AD. The risk allele T and T carriers of ERG rs2836411 are associated with anemia and AD presence. Of note, anemia may mediate the association between ERG rs2836411 polymorphism and the susceptibility to AD. This finding will help develop future screening and interventional techniques that specifically target the ERG rs2836411 polymorphism, thereby strengthening the prevention and treatment of AD.
Limitations
Certainly, this study has several limitations. First, only a very few cases of TT homozygous mutations were detected in our study population due to sample size constraints, resulting in an inaccurate analysis of TT genotypes. Therefore, we merged heterozygous and homozygous mutations and focused on analyzing the differences between CC and T carriers (CT + TT) in the dominant model. Nevertheless, we were able to correctly identify the impact of the ERG rs2836411 polymorphism on the anemia and AD susceptibility by discerning the differences between patients with or without the risk allele T. Second, due to the lack of data such as reticulocytes and ferritin, we can only categorize anemia morphologically in this study. The relationship between anemia of different causes/types and AD needs to be further clarified in future research. Third, given the nature of case-control study, we can only calculate the odds ratio, not the risk ratio, to explore the association between exposure factors and outcome events. Future prospective studies are required to validate the potential risk factors identified in the current study. Fourth, although we have confirmed a significant association between the ERG rs2836411 polymorphism and the presence of anemia and AD, our study’s statistical power falls slightly short of the conventional threshold of 0.8, with a power of 0.76. Future studies with larger sample size are required to confirm the observed associations. Last, we cannot conclude their causal connection or determine whether this association is mediated by ERG from endothelial or hematopoietic sources. Future experimental studies using transgenic mice with inducible hematopoietic or endothelial Erg depletion are warranted to demonstrate the causal connection between ERG rs2836411 polymorphism and the susceptibility to AD as well as the underlying mechanisms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Yazhou Wu from the Statistics Teaching and Research Office of the Army Medical University for advice on statistical analyses.
Author contributions
Conceptualization, J.J., Y.W. ; methodology, J.J., Y.W., G.L. (Gaoshan Li) and F. Z. (Fangzheng Zeng); software, G.L. (Gaoshan Li) and F. Z. (Fangzheng Zeng); validation, G.L. (Gaoshan Li); formal analysis, Y.W. and G.L. (Gaoshan Li); investigation, K.L. (Kunyan Li); resources, W.P. (Wenxu Pan); data curation, M.Z. (Mingle Zhang) and H.Y.; writing—original draft preparation, Y.W. and G.L. (Gaoshan Li); writing—review and editing, J.J. and Y.W.; visualization, Y.W. and G.L.; supervision, J.J.; project administration, J.J.; funding acquisition, J.J. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (No.82100288), Chongqing Doctoral Research Fund (No. CSTB2022BSXM-JCX0023), Chongqing Innovation Support Program for Returned Overseas Scholars (No. cx2020010), Young Doctoral Talent Program (No. 2022YQB009), and Chongqing Talent Program (No. CQYC201903087).
Data availability
SNP datasets of all participants have been deposited in the China National Center for Bioinformation Database (https://ngdc.cncb.ac.cn/omix, Identifier: OMIX005116).
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Xinqiao Hospital, Army Medical University (2022-059-03). All eligible patients supplied written informed consent, and the study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Wang, Gaoshan Li and Fangzheng Zeng contributed equally to this work.
References
- 1.Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet. 2015;385(9970):800–11. [DOI] [PubMed] [Google Scholar]
- 2.Lawler PR, Filion KB, Dourian T, Atallah R, Garfinkle M, Eisenberg MJ. Anemia and mortality in acute coronary syndromes: a systematic review and meta-analysis. AM HEART J. 2013;165(2):143–53. [DOI] [PubMed] [Google Scholar]
- 3.Gao Z, Qin Z, An Z, Hou C, Wang L, Jin J. Prognostic value of preoperative hemoglobin levels for long-term outcomes of Acute Type B aortic dissection post-thoracic endovascular aortic repair. FRONT CARDIOVASC MED. 2020;7:588761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin K, Willie-Permor D, Zarrintan S, Dakour-Aridi H, Ramirez JL, Iannuzzi JC, Naazie I, Malas MB. Anemia is associated with higher mortality and morbidity after thoracic endovascular aortic repair. J VASC SURG. 2023;77(2):357–65. [DOI] [PubMed] [Google Scholar]
- 5.Marsman J, Gimenez G, Day RC, Horsfield JA, Jones GT. A non-coding genetic variant associated with abdominal aortic aneurysm alters ERG gene regulation. HUM MOL GENET. 2020;29(4):554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones GT, Tromp G, Kuivaniemi H, Gretarsdottir S, Baas AF, Giusti B, Strauss E, van Hof T, Webb FNG, Erdman TR. Meta-analysis of Genome-Wide Association Studies for Abdominal aortic aneurysm identifies four New Disease-specific risk loci. CIRC RES. 2017;120(2):341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah AV, Birdsey GM, Randi AM. Regulation of endothelial homeostasis, vascular development and angiogenesis by the transcription factor ERG. VASC PHARMACOL. 2016;86:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperone A, Dryden NH, Birdsey GM, Madden L, Johns M, Evans PC, Mason JC, Haskard DO, Boyle JJ, Paleolog EM, et al. The transcription factor erg inhibits vascular inflammation by repressing NF-κB activation and proinflammatory gene expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31(1):142–50. [DOI] [PubMed] [Google Scholar]
- 9.Loughran SJ, Kruse EA, Hacking DF, de Graaf CA, Hyland CD, Willson TA, Henley KJ, Ellis S, Voss AK, Metcalf D, et al. The transcription factor erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. NAT IMMUNOL. 2008;9(7):810–9. [DOI] [PubMed] [Google Scholar]
- 10.Shah AV, Birdsey GM, Peghaire C, Pitulescu ME, Dufton NP, Yang Y, Weinberg I, Osuna Almagro L, Payne L, Mason JC et al. The endothelial transcription factor ERG mediates Angiopoietin-1-dependent control of notch signalling and vascular stability. NAT COMMUN 2017, 8(1). [DOI] [PMC free article] [PubMed]
- 11.Walker L, Carlson A, Tan-Pertel HT, Weinmaster G, Gasson J. The notch receptor and its ligands are selectively expressed during hematopoietic development in the mouse. Stem Cells. 2001;19(6):543–52. [DOI] [PubMed] [Google Scholar]
- 12.Bunn HF, Erythropoietin. CSH PERSPECT MED. 2013;3(3):a011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garimella PS, Katz R, Patel KV, Kritchevsky SB, Parikh CR, Ix JH, Fried LF, Newman AB, Shlipak MG, Harris TB et al. Association of Serum Erythropoietin with Cardiovascular Events, kidney function decline, and Mortality. Circulation: Heart Fail 2016, 9(1). [DOI] [PMC free article] [PubMed]
- 14.Grote Beverborg N, van der Wal HH, Klip IT, Voors AA, de Boer RA, van Gilst WH, van Veldhuisen DJ, Gansevoort RT, Hillege HL, van der Harst P, et al. High serum erythropoietin levels are related to heart failure development in subjects from the general population with albuminuria: data from PREVEND. EUR J HEART FAIL. 2016;18(7):814–21. [DOI] [PubMed] [Google Scholar]
- 15.El AM, El KA, Asserraji M. Partially thrombosed aneurysm of the abdominal aorta: unusual cause of chronic inflammation and resistance to recombinant human erythropoietin. INDIAN J NEPHROL. 2014;24(1):38–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M, Sui W, Cheng C, Xue F, Tian Z, Cheng J, Zhang J, Zhang T, Zhang J, Wang W et al. Erythropoietin promotes abdominal aortic aneurysms in mice through angiogenesis and inflammatory infiltration. SCI TRANSL MED 2021, 13(603). [DOI] [PubMed]
- 17.Wang X, Wu Z, Chen Y, Zhu J, Dong X, Fu C, Jiang Q. Increased prevalence and incidence of anemia among adults in transforming rural China: two cross-sectional surveys. BMC Public Health 2015, 15(1). [DOI] [PMC free article] [PubMed]
- 18.Ford J. Red blood cell morphology. INT J LAB HEMATOL. 2013;35(3):351–7. [DOI] [PubMed] [Google Scholar]
- 19.Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22(15):1928–9. [DOI] [PubMed] [Google Scholar]
- 20.Honda Y, Watanabe T, Otaki Y, Tamura H, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Miyamoto T, Shibata Y, et al. Gender differences in the impact of anemia on subclinical myocardial damage and cardiovascular mortality in the general population: the Yamagata (Takahata) study. INT J CARDIOL. 2018;252:207–12. [DOI] [PubMed] [Google Scholar]
- 21.Gan T, Hu J, Liu W, Li C, Xu Q, Wang Y, Lu S, Aledan AKO, Wang Y, Wang Z. Causal Association between Anemia and Cardiovascular Disease: a 2-Sample bidirectional mendelian randomization study. J AM HEART ASSOC 2023, 12(12). [DOI] [PMC free article] [PubMed]
- 22.Gorla R, Tsagakis K, Horacek M, Mahabadi A, Kahlert P, Jakob H, Bossone E, Erbel R, Jánosi RA. Impact of Preoperative Anemia and Postoperative Hemoglobin Drop on the incidence of Acute kidney Injury and In-Hospital mortality in patients with type B Acute aortic syndromes undergoing thoracic endovascular aortic repair. VASC ENDOVASC SURG. 2017;51(3):131–8. [DOI] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. NEW ENGL J MED. 2011;364(7):656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmann G, Tschöp M, Fischer R, Bidlingmaier C, Riepl R, Tschöp K, Hautmann H, Endres S, Toepfer M. HIGH ALTITUDE INCREASES CIRCULATING INTERLEUKIN-6, INTERLEUKIN-1 RECEPTOR ANTAGONIST AND C-REACTIVE PROTEIN. Cytokine. 2000;12(3):246–52. [DOI] [PubMed] [Google Scholar]
- 25.He S, Sun S, Chen A, Lv S, Qiu C, Wei M, Liu W, Liu H, Zhang L, Ren D. Hypoxia regulates cytokines expression and neutrophils migration by ERK signaling in zebrafish. FISH SHELLFISH IMMUN. 2022;125:212–9. [DOI] [PubMed] [Google Scholar]
- 26.Rombouts KB, van Merrienboer TAR, Ket JCF, Bogunovic N, van der Velden J, Yeung KK. The role of vascular smooth muscle cells in the development of aortic aneurysms and dissections. EUR J CLIN INVEST. 2022;52(4):e13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin Z, Han H, Yan X, Zheng Q. Research Progress on the pathogenesis of aortic dissection. CURR PROB Cardiol. 2023;48(8):101249. [DOI] [PubMed] [Google Scholar]
- 28.Prokop EK, Palmer RF, Wheat MJ. Hydrodynamic forces in dissecting aneurysms. In-vitro studies in a Tygon model and in dog aortas. CIRC RES. 1970;27(1):121–7. [DOI] [PubMed] [Google Scholar]
- 29.Rajagopal K, Bridges C, Rajagopal KR. Towards an understanding of the mechanics underlying aortic dissection. BIOMECH MODEL MECHAN. 2007;6(5):345–59. [DOI] [PubMed] [Google Scholar]
- 30.DUKE M, ABELMANN WH. The hemodynamic response to chronic Anemia. Circulation. 1969;39(4):503–15. [DOI] [PubMed] [Google Scholar]
- 31.Metivier F, Marchais SJ, Guerin AP, Pannier B, London GM. Pathophysiology of anaemia: focus on the heart and blood vessels. NEPHROL DIAL TRANSPL. 2000;15(suppl3):14–8. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Chang Y, Jiang P, Ma Y, Yuan Q, Ma X. Association of gene polymorphisms in FBN1 and TGF-beta signaling with the susceptibility and prognostic outcomes of Stanford type B aortic dissection. BMC MED GENOMICS. 2022;15(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang W, Wang X, Gao P, Li F, Lu K, Tan X, Zheng S, Pei W, An M, Li X et al. Association of IL1R1 coding variant with plasma-level soluble ST2 and risk of aortic dissection. FRONT CARDIOVASC MED 2021,8:710425. [DOI] [PMC free article] [PubMed]
- 34.Ng AP, Loughran SJ, Metcalf D, Hyland CD, de Graaf CA, Hu Y, Smyth GK, Hilton DJ, Kile BT, Alexander WS. Erg is required for self-renewal of hematopoietic stem cells during stress hematopoiesis in mice. Blood. 2011;118(9):2454–61. [DOI] [PubMed] [Google Scholar]
- 35.Schafer CM, Martin-Almedina S, Kurylowicz K, Dufton N, Osuna-Almagro L, Wu M, Johnson CF, Shah AV, Haskard DO, Buxton A, et al. Cytokine-mediated degradation of the transcription factor ERG impacts the pulmonary vascular response to systemic inflammatory challenge. Arterioscler Thromb Vasc Biol. 2023;43(8):1412–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birdsey GM, Shah AV, Dufton N, Reynolds LE, Osuna Almagro L, Yang Y, Aspalter IM, Khan ST, Mason JC, Dejana E, et al. The endothelial transcription factor ERG promotes Vascular Stability and Growth through Wnt/β-Catenin signaling. DEV CELL. 2015;32(1):82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newhall DA, Oliver R, Lugthart S. Anaemia: a disease or symptom. NETH J MED. 2020;78(3):104–10. [PubMed] [Google Scholar]
- 38.Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siripin D, Kheolamai P, U-Pratya Y, Supokawej A, Wattanapanitch M, Klincumhom N, Laowtammathron C, Issaragrisil S. Transdifferentiation of erythroblasts to megakaryocytes using FLI1 and ERG transcription factors. THROMB HAEMOSTASIS. 2017;114(09):593–602. [DOI] [PubMed] [Google Scholar]
- 40.Carmichael CL, Metcalf D, Henley KJ, Kruse EA, Di Rago L, Mifsud S, Alexander WS, Kile BT. Hematopoietic overexpression of the transcription factor erg induces lymphoid and erythro-megakaryocytic leukemia. Proc Natl Acad Sci. 2012;109(38):15437–42. [DOI] [PMC free article] [PubMed]
- 41.Angelos MG, Abrahante JE, Blum RH, Kaufman DS. Single cell resolution of human hematoendothelial cells defines transcriptional signatures of Hemogenic Endothelium. Stem Cells. 2018;36(2):206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michel J, Jondeau G, Milewicz DM. From genetics to response to injury: vascular smooth muscle cells in aneurysms and dissections of the ascending aorta. CARDIOVASC RES. 2018;114(4):578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
SNP datasets of all participants have been deposited in the China National Center for Bioinformation Database (https://ngdc.cncb.ac.cn/omix, Identifier: OMIX005116).