Abstract
Chemokines are low-molecular-weight chemotactic cytokines that have been shown to play a central role in the perivascular transmigration and accumulation of specific subsets of leukocytes at sites of tissue damage. Using in situ hybridization (ISH), we investigated the mRNA induction of macrophage inflammatory protein 2 (MIP-2), MIP-1α, monocyte chemoattractant protein 1 (MCP-1), and RANTES. Challenge of infant rats’ brains with Haemophilus influenzae type b intraperitoneally resulted in the time-dependent expression of MIP-2, MIP-1α, MCP-1, and RANTES, which was maximal 24 to 48 h postinoculation. Immunohistochemistry showed significant increases in neutrophils and macrophages infiltrating the meninges, the ventricular system, and the periventricular area. The kinetics of MIP-2, MIP-1α, MCP-1, and RANTES mRNA expression paralleled those of the recruitment of inflammatory cells and disease severity. Administration of anti-MIP-2 or anti-MIP-1α antibodies (Abs) resulted in significant reduction of neutrophils. Administration of anti-MCP-1 Abs significantly decreased macrophage infiltration. Combined studies of ISH and immunohistochemistry showed that MIP-2- and MIP-1α-positive cells were neutrophils and macrophages. MCP-1-positive cells were neutrophils, macrophages, and astrocytes. Expression of RANTES was localized predominantly to resident astrocytes and microglia. The present study indicates that blocking of MIP-2 or MIP-1α bioactivity in vivo results in decreased neutrophil influx. These data are also the first demonstration that the C-C chemokine MIP-1α is involved in neutrophil recruitment in vivo.
The accumulation of leukocytes at sites of inflammation is induced by the local production and secretion of chemotactic ligands by a wide variety of stimulated cell types. Recently, several host-derived cytokines (chemokines) have been identified that stimulate chemotaxis in vitro and elicit the accumulation of various types of inflammatory cells in vivo (21).
A variety of mediators contribute to early pathophysiological alterations in bacterial meningitis. Meningeal inflammatory reaction is initiated when more than 105 bacteria/ml are present in the cerebrospinal fluid (CSF) (35). This process is characterized by increasing levels of proinflammatory cytokines (particularly interleukin 1β [IL-1β], IL-6, and tumor necrosis factor alpha) (8, 16, 23, 35, 36) and leukocyte infiltration of the subarachnoid space (24, 30). Tumor necrosis factor alpha and IL-1 are known to induce adhesion molecules of the selectin family on the surface of endothelial cells to which leukocytes bind and roll along the vessel wall (15). It is still unclear how leukocytes leave the circulation and migrate through the tight endothelial cell barrier of the brain vessels, designated the blood-brain barrier (10). Two major groups of molecules are thought to be relevant during leukocyte-endothelial cell interactions preceding the trafficking of leukocytes across the blood-brain barrier: (i) cellular adhesion molecules of endothelial cells and their counterreceptors on leukocytes induce attachment of circulating blood cells to the vessel wall, and (ii) chemokines activate and attract specific leukocyte subsets, leading to extravasation and accumulation of these cells in the injured or inflamed tissue (32). Chemokines form a large family of structurally homologous proteins with molecular masses of between 8 and 13 kDa. They are involved in the inflammatory host response to foreign pathogens by attracting and stimulating leukocytes (1, 21).
Members of the chemokine gene superfamily of cytokines have homologous sequences and a highly conserved cysteine motif in their primary amino acid structure. Chemokines can be divided into four groups depending on whether the first pair of cysteines is separated (C-X-C) or not (C-C) by an intervening amino acid, whether the second cysteine is missing (C), or whether the first pair of cysteines is separated by three amino acids (C-X3-C) (2, 22). In general, with respect to their chemotactic effects, the biologic targets of the members of the three chemokine subfamilies can be broadly divided into three major categories. The C-X-C or α subfamily, represented by IL-8 and macrophage inflammatory protein 2 (MIP-2), attracts mainly polymorphonuclear granulocytes, whereas members of the chemokine β subfamily, including MIP-1α, MIP-1β, monocyte chemoattractant protein 1 (MCP-1), and RANTES, are potent chemotactic agents for monocytes, lymphocytes, and other cell types, such as basophils and eosinophils (1, 25, 26, 33). Finally, lymphotactin, the only C chemokine known to date, is principally chemotactic for CD8+ T lymphocytes and does not appear to act on other myeloid cells (13), while the C-X3-C chemokine fractalkine or neurotactin has been reported to act as a chemoattractant for T cells, monocytes, and neutrophils (2, 22). The chromosome locations of the corresponding genes, i.e., human chromosome 4 for the α subfamily and chromosome 17 for the β subfamily, are distinct (21, 38). Based on amino acid sequence, no direct homology between human and rat IL-8 has been found. However, rat MIP-2, which is a homologue of the human C-X-C chemokine melanoma growth-stimulatory activity (GRO), has been functionally characterized as a major polymorphonuclear granulocyte-activating factor and chemoattractant that parallels the actions of IL-8 in humans (37, 40).
In the present study on experimental bacterial meningitis we analyzed the mRNA expression of certain members of two chemokine subfamilies, namely, MIP-1α, MCP-1, RANTES, and MIP-2. We have determined the functional role of the α chemokine MIP-2 and the β chemokines MIP-1α and MCP-1 in the cascade of events which mediate distinct leukocyte population migration into the subarachnoid space during experimental bacterial meningitis. In addition, we studied the cellular sources of MIP-1α, MCP-1, RANTES, and MIP-2 by combined in situ hybridization and immunohistochemical staining.
MATERIALS AND METHODS
Infecting organism.
The organism used in all experiments was Haemophilus influenzae type b (Hib) strain LCR 528. It consists of the type b encapsulated strain as confirmed by slide agglutination with type-specific antiserum and was originally obtained from the CSF of a patient with Hib meningitis. It was grown in brain heart infusion broth supplemented with 5% Fildes enrichment medium to late log phase and then frozen at −70°C in Trypticase soy broth with 10% glycerol (pH 7.3) until use. An overnight growth was subcultured on a chocolate agar plate and allowed to grow for 8 h in the late log phase to facilitate maximal capsule expression in the challenge inocula and then was centrifuged, washed, and resuspended in phosphate-buffered saline (PBS) to an approximate concentration of 105 CFU per ml. The inoculum was checked for purity and density by quantitative subcultures.
Infant rat model of H. influenzae meningitis.
Nursing Sprague-Dawley rat pups with their dam were purchased, and 98 infected and 24 control pups were studied. The pups (7 days old) were used as described previously (28). Meningitis was induced by intraperitoneal (i.p.) inoculation with a single given dose of Hib in 100 μl (see above). Control animals received an identical injection of sterile PBS. Pups were returned to their mothers. At given evaluation time points (12 h, 24 h, 48 h, 72 h, 96 h, and 7 days), animals were sacrificed by i.p. injection with pentobarbital (200 mg/kg of body weight) and their brains were dissected.
Immunohistochemical phenotyping and quantitation of leukocytes.
Brains were snap-frozen in liquid nitrogen. Cryostat sections, 10 μm thick, from brains of Hib-infected and uninfected control pups were mounted on gelatin-coated glass slides, air dried, and then fixed in acetone at 4°C for 5 min. Fixed sections were stained with mouse monoclonal antibodies (Abs) directed against CD5 (a marker for T cells), granulocytes, ED1 (a marker for macrophages), OX42 (anti-complement receptor type 3, a marker for microglia) (all from Biosource International, Camarillo, Calif.), and glial fibrillary acid protein (GFAP) (Boehringer Biochemica, Mannheim, Germany). An avidin-biotin method was used, all incubations were carried out under humidified conditions, and slides were washed three times between steps for 5 min each in PBS. First, endogenous peroxidase was blocked by incubation for 30 min in methanol containing 0.3% hydrogen peroxide. After preabsorption with normal serum, sections were incubated with primary antibody overnight at 4°C. After washing, the sections were overlaid for 1 h with biotinylated horse anti-mouse Abs (Southern Biotechnology, Birmingham, Ala.) followed by avidin-biotin complex (ABC Vectstain Elite kit; Vector, Burlingame, Calif.) for 30 min. Peroxidase activity was visualized with 3,3′-diaminobenzidine (DAB kit; Vector) as the substrate, with a hematoxylin counterstain. Omission of the primary Ab served as a negative control. Specificity of the staining was also controlled on sections of peripheral lymphoid organs. The tissue areas were measured by a Seescan (Cambridge, United Kingdom) image analysis system, and the numbers of stained cells per 100 mm2 of tissue area were calculated.
Measurement of mRNA expression by in situ hybridization.
Cryostat sections, 10 μm thick, were thaw mounted onto electrically charged glass slides (ProbeOn slides; Fisher Scientific, Pittsburgh, Pa.), which were stored with silica in sealed boxes at −20°C until hybridization. In situ hybridization was performed as described for tissue sections (7). Synthetic oligonucleotide probes (Scandinavian Gene Synthesis AB, Köping, Sweden) were labelled by using 35S-deoxyadenosine-5′-α-(thio)-triphosphate (New England Nuclear, Cambridge, Mass.) with terminal deoxynucleotidyl transferase (Amersham, Little Chalfont, United Kingdom). To increase the sensitivity of the method, a mixture of four different ∼48-bp oligonucleotide probes was used. The oligonucleotide sequences were obtained from GenBank, and probes were designed by using MacVector software (Table 1). After hybridization, slides were rinsed three times for 15 min at 55°C in 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), allowed to come to room temperature, dipped in distilled water, dehydrated through a gradient ethanol series (60, 70, and 95%), air dried, dipped in Kodak NTB2 emulsion, and exposed at 4°C for 15 days, depending on the age of the probe. After development in Kodak D19, the slides were stained with cresyl violet and mounted with Entellan (Merck, Darmstadt, Germany). Coded slides were examined by dark-field microscopy at a magnification of ×10. Cells were judged as positive when expressing more than 12 grains with a star-like distribution over their cytoplasm. In cells judged negative, the number of grains was mostly 0 to 2 per cell, and the grains were scattered randomly over the cell and not distributed in a star-like fashion. The cellular distribution of the grains was always checked under light microscopy at a magnification of ×20 and/or ×40. There were no difficulties in differentiating between chemokine mRNA-positive and -negative cells. Control probes used in parallel with the chemokine probes on tissue sections produced a weak background signal without revealing any positive cells (Table 1). Results are expressed as numbers of cells per 100-mm2 tissue section. The tissue section areas were measured by image analysis.
TABLE 1.
Rat chemokine probes used in this study
| Target (accession no.) and probe (designation) |
Sequence |
|---|---|
| RANTES (U06436) | |
| Probe 1 (rRANTES-21L30) | 5′ GCT GCA ACG AGG ATG ACG GTG AGG GTA GCA 3′ |
| Probe 2 (rRANTES-83L30) | 5′ CAA AGC AGC AGG GAG TGG TGT CCG AGC CAT 3′ |
| Probe 3 (rRANTES-128L30) | 5′ AAT ACT CCT TCA CGT GGG CAC GGG GCA GTG 3′ |
| Control probe (rRantes-192U30) | 5′ CGT CTT TGT CAC TCG AAG GAA CCG CCA AGT 3′ |
| MCP1 (M57441) | |
| Probe 1 (rMCP1-44L30) | 5′ ACA GGC CCA GAA GCG TGA CAG AGA CCT GCA 3′ |
| Control probe | 5′ TGC AGG TCT CTG TCA CGC TTC TGG GCC TGT 3′ |
| Probe 2 (rMCP1-159L29) | 5′ CCA GCC GAC TCA TTG GGA TCA TCT TGC CA 3′ |
| Control probe | 5′ TGG CAA GAT GAT CCC AAT GAG TCG GCT GG 3′ |
| MIP-1α (U06435) | |
| Probe 1 (rMIP-1α-21L29) | 5′ GCG CCA TGG TGC AGA GAA GAA CAG CAA GG 3′ |
| Control probe | 5′ CCT TGC TGT TCT TCT CTG CAC CAT GGC GC 3′ |
| Probe 2 (rMIP-1α-78L30) | 5′ AAG CAG CAG GCA GTC GGG GTG TCA GCT CCA 3′ |
| Control probe | 5′ TGG AGC TGA CAC CCC GAC TGC CTG CTG CTT 3′ |
| Probe 3 (rMIP-1α-201L30) | 5′ TTG GGG TCA GCG CAG ATG TGC CGG TTT CTC 3′ |
| Control probe | 5′ GAG AAA CCG GCA GAT CTG CGC TGA CCC CAA 3′ |
| MIP2 (S77604) | |
| Probe 1 (rMIP2-48L30) | 5′ GCA GAA GGC GGC CAC AAG CAG GAG GAG A 3′ |
| Control probe | 5′ TCC TCA ATG CTG TAC TGG TCC TGC TCC TCC 3′ |
| Probe 2 (rMIP2-205L30) | 5′ GTG GCT ATG ACT TCT GTC TGG GCG CAG TGG 3′ |
| Control probe | 5′ CCA CTG CGC CCA GAC AGA AGT CAT AGC CAC 3′ |
| Probe 3 (rMIP2-268L30) | 5′ TGG ACG ATC CTC TGA ACC AAG GGG GCT TCA 3′ |
| Control probe | 5′ TGA AGC CCC CTT GGT TCA GAG GAT CGT CCA 3′ |
Blocking experiments.
Experiments were performed to modulate disease by the administration of mediators that neutralize or modify chemokine function. Thus, in separate experiments, groups of pups (n = 72) received daily i.p. doses of either polyclonal rabbit anti-rat MIP-2, polyclonal rabbit anti-rat MIP-1α, or polyclonal rabbit anti-rat MCP-1 (all from Biosource International) 30 min before Hib infection. Anti-MIP-2, anti-MIP-1α, and anti-MCP-1 were administered at 5 μg/pup/day. Controls included pups given normal rabbit immunoglobulin (Ig) (Serotec, Oxford, United Kingdom) at 5 μg/pup/day. These blocking reagents were given at stated doses from day 0 to day 7 p.i. Pups were then killed on days 3 and 7 p.i., and brains were removed for in situ hybridization and immunohistological analyses, as described above.
Combined study of in situ hybridization and immunohistochemical staining.
At 48 h p.i., sections were subjected to in situ hybridization for MIP-2, MIP-1α, MCP, and RANTES mRNA and histological staining with cell type-specific Abs. After in situ hybridization, slides were rinsed three times for 15 min at 55°C in 1× SSC; allowed to come to room temperature; immunohistochemically stained for granulocyte, ED1, CD5, OX42, and GFAP Abs as described above; and then dehydrated in 60, 95, and 100% ethanol and emulsified.
Statistical analysis.
The nonparametric Mann-Whitney test was used to evaluate the statistical significance of differences between treated and isotype control-treated groups of Hib-inoculated rats.
Ethics.
The studies were reviewed by the Animal Ethics Committee of the Karolinska Institute at Huddinge University Hospital (no. S 111/95).
RESULTS
Infant rats with Hib infection were observed to have poor weight, tremors, and hair ruffling. Prior to death, animals stopped nursing, lay on their sides, and were unresponsive to painful stimuli. The peak incidence of deaths of infant rats when given a lethal dose (105 CFU/ml) was at 24 to 96 h, with occasional deaths occurring after that time. Time points were chosen which corresponded to onset of early clinical illness (12 h postinoculation [p.i.]), maximal clinical illness (24 to 96 h p.i.), and recovery stage in animals surviving the acute infection (day 7 p.i.).
Characterization of inflammatory infiltrate.
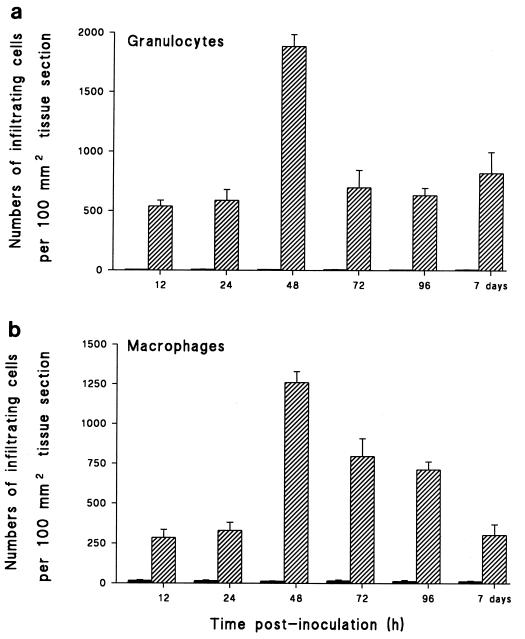
Infiltrating neutrophils, mononuclear phagocytes, and lymphocytes were identified at different time points during the course of meningitis by immunohistochemical staining of frozen sections. Increases in both neutrophils and macrophages/monocytes, but not lymphocytes, were observed 12 h after Hib inoculation. However, by 48 h p.i., neutrophils rather than cells of the macrophage lineage dominated the subarachnoid space and ventricular infiltrate (Fig. 1). Peak inflammatory infiltration was seen at 48 h p.i., with a decline in numbers occurring by day 7 p.i. Staining with lymphocyte-specific markers at various time points revealed the absence of CD5+ T cells.
FIG. 1.
Inoculation of Hib i.p. induced an increase in the number of leukocytes within brain tissue. Sections were prepared from brains of PBS-inoculated controls (closed bars) or Hib-inoculated infant rats (hatched bars) at various time points after disease induction and stained with Abs specific for granulocytes (a) and macrophages (b). Numbers of positively stained cells per 100 mm2 of surface area were determined. Data are given as mean numbers of cells taken from groups of six rats + standard deviations (error bars).
Detection of MIP-2, MIP-1α, MCP-1, and RANTES mRNA in the brains of infant rats with Hib meningitis.
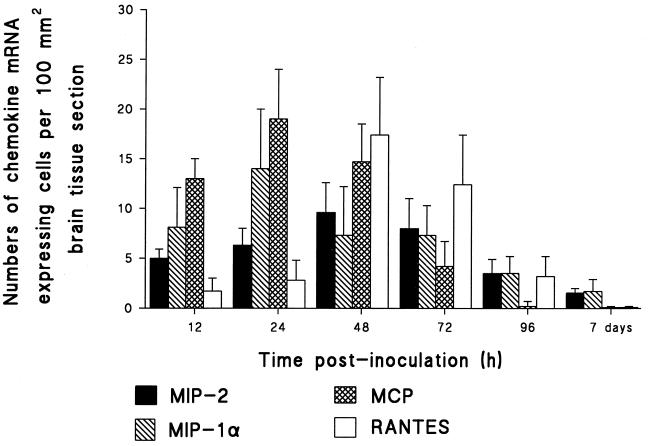
Brain tissue obtained from Hib- and PBS-inoculated rats between 12 h and 7 days p.i. was analyzed by in situ hybridization for transcripts of MIP-2, MIP-1α, MCP-1, and RANTES. No chemokine mRNA was detected in the brain tissue of PBS-inoculated rats. In the brain tissue of Hib-infected rats, a slight increase of MIP-2 mRNA was already apparent at 12 h p.i. Levels remained similarly elevated at 24 h, and maximal induction was registered at 48 h p.i., thereafter decreasing up to day 7 p.i. but still remaining detectable. MIP-1α exhibited marked induction at 12 h p.i., reaching maximal levels at 24 h. Levels declined and remained detectable up to day 7 p.i. Elevated induction of MCP-1 was recorded at 12 h p.i., the peak transcription was recorded at 24 h p.i., and remaining high levels were recorded at 48 h p.i. Thereafter, levels declined but remained recordable until 96 h p.i. Very low levels of RANTES mRNA-expressing cells were detected at 12 and 24 h p.i. Maximal induction was obtained at 48 h p.i., and decreasing levels were recorded up to 96 h p.i. When brains from infected animals were analyzed with the sense chemokine probe, no hybridization signals became apparent (Fig. 2).
FIG. 2.
MIP-2, MIP-1α, MCP-1, and RANTES mRNA expression in brain. Results are given as mean numbers of cells expressing mRNA for MIP-2, MIP-1α, MCP-1, and RANTES per 100 mm2 of surface area, detected by in situ hybridization as described in Materials and Methods (error bars, standard deviations). Brain sections were obtained from Hib-inoculated and control rats at 12 h, 24 h, 48 h, 72 h, 96 h, and 7 days p.i.
Significance of MIP-2 blocking in bacterial meningitis.
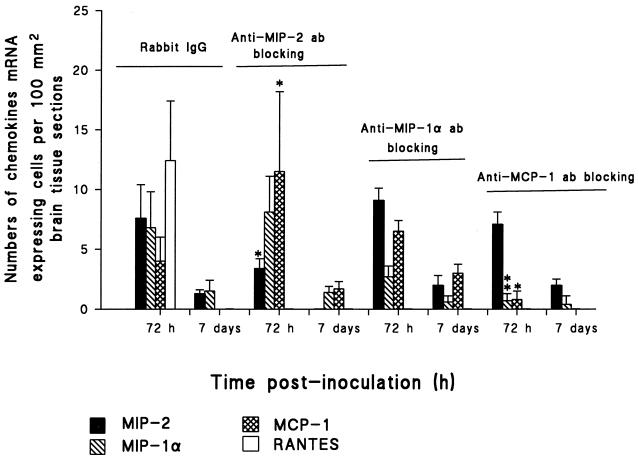
To discern the contribution of MIP-2 to the acute phase of meningeal inflammation, we next treated the animals in vivo with polyclonal rabbit anti-rat MIP-2. Days 3 and 7 p.i. were chosen to determine the effect of blocking MIP-2 on inflammatory infiltrate and chemokine mRNA expression. Thus, 5 μg of anti-MIP-2 was administered daily until day 3 and day 7. First anti-MIP-2 Ab significantly attenuated the influx of granulocytes and macrophages to the leptomeninges and to the periventricular and ventricular system (Fig. 3 to 5). In situ hybridization performed on brain tissue of anti-MIP-2 Ab-treated animals revealed that these animals had significantly lower levels of MIP-2 as well as RANTES mRNA-expressing cells than did rabbit Ig-treated control animals. Interestingly, MCP-1 mRNA-expressing cells in the brain of anti-MIP-2 Ab-treated rats continued to be significantly higher than in the rabbit Ig-treated control rats. However, there was no difference in levels of MIP-1α mRNA-expressing cells in the anti-MIP-2 Ab-treated animals and rabbit Ig-treated control animals (Fig. 6). The experiments were repeated with a higher dose of anti-MIP-2 Ab (10 μg/pup/day) without additional benefit (data not shown).
FIG. 3.
Abs to MIP-2, MIP-1α, and MCP-1 reduce granulocyte and macrophage infiltration. Four groups with six infant rats each received either anti-MIP-2, anti-MIP-1α, or MCP-1 Ab (5 μg/day) or isotype control Ab (5 μg/day) i.p. 30 min before Hib inoculation. Rats then received i.p. injections with 100 μl containing 105 CFU of Hib per ml. Rats were treated daily with 5 μg of rabbit Ig or rabbit anti-rat MIP-2, MIP-1α, or MCP-1 Ab per rat. Rats were killed 72 h and 7 days p.i. Numbers of granulocytes and macrophages were determined in immunohistochemically stained sections per 100 mm2 of surface area. For each panel, the significance of differences between treated and isotype control-treated groups of Hib-inoculated rats was determined by Student’s t test. Means and standard deviations (error bars) are depicted. ∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.05.
FIG. 5.
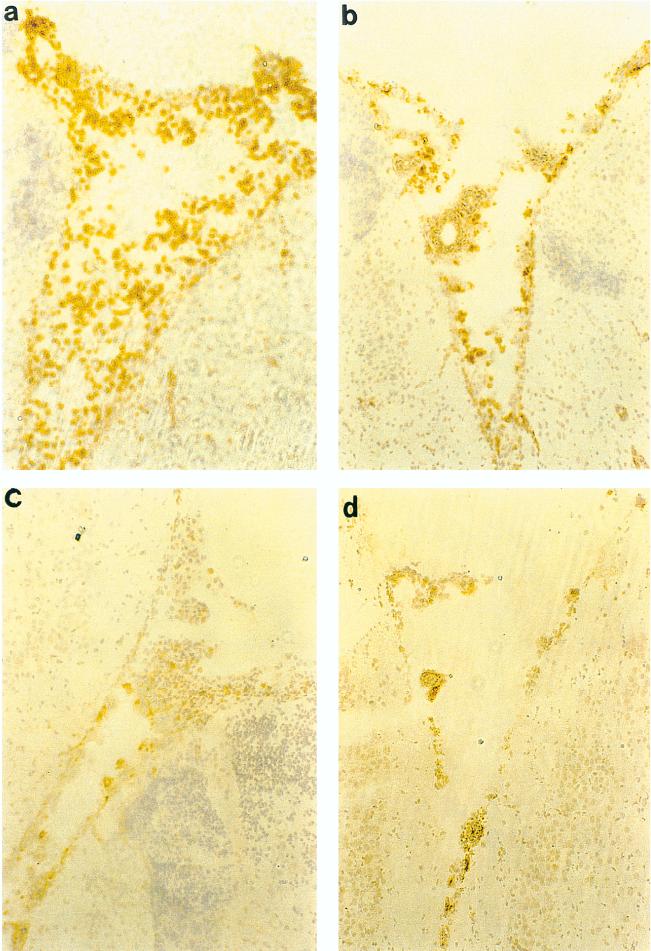
Effect of antichemokine Abs on granulocytes infiltrating the brains of Hib-infected rats as shown by immunohistochemistry. Shown are representative fields (obtained 72 h p.i.) from animals treated with rabbit Ig as control Ab (a), rabbit polyclonal anti-MIP-2 (b), rabbit polyclonal anti-MIP-1α (c), and rabbit polyclonal anti-MCP-1 (d). (a) Control Ab-treated animals showed diffuse granulocyte infiltration in the third ventricle. Anti-MIP-2 and anti-MIP-1α-treated animals showed much reduced granulocyte infiltration. Animals treated with anti-MCP-1 exhibited less diffuse granulocyte infiltration. Staining was performed on 10-μm-thick cryosections as described in Materials and Methods, with immunoperoxidase and hematoxylin. Magnification, ×400.
FIG. 6.
Effect of MIP-2, MIP-1α, and MCP-1 Abs on chemokine mRNA expression. Four groups of six infant rats received either anti-MIP-2, anti-MIP-1α, and anti-MCP-1 Ab (5 μg/day) or isotype control Ab (5 μg/day) i.p. 30 min before Hib inoculation. Rats then received i.p. injections with 100 μl containing 105 CFU of Hib per ml. Results are given as mean numbers of cells expressing mRNA for MIP-2, MIP-1α, MCP-1, and RANTES per 100 mm2 of surface area, detected by in situ hybridization as described in Materials and Methods (error bars, standard deviations). Brain sections were obtained from anti-MIP-2-treated and isotype control-inoculated groups at 72 h and 7 days p.i. For each panel, the significance of differences between treated and untreated groups of Hib-inoculated rats was determined by Student’s t test. ∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.05.
Blocking experiments with anti-MIP-1α and anti-MCP-1.
A neutralizing polyclonal rabbit anti-rat MIP-1α and a polyclonal rabbit anti-rat MCP-1 were used to assess the contribution of these chemokines to the course of bacterial meningitis. Experiments were conducted exactly as described above for anti-MIP-2 Ab with 5 μg of anti-MIP-1α or 0.5 μg of anti-MCP-1. Rabbit Ig was used as an isotype-matched control antibody. Isotype-matched control antibody had no effect upon the development of the disease. Immunohistochemical phenotyping revealed that anti-MIP-1α significantly decreased the recruitment of granulocytes and macrophages to the ventricular system and the leptomeninges (Fig. 3 to 5). In the brain tissue of anti-MIP-1α Ab-treated animals, MIP-2 mRNA expression was increased on days 3 and 7 p.i. compared to that in the isotype-matched control antibody-treated animals. This difference did not, however, reach statistical significance. Anti-MIP-1α Ab administration suppressed cells expressing mRNA of MIP-1α (not significant) and RANTES (Fig. 6). Interestingly, levels of MCP-1 mRNA-expressing cells in the brain of anti-MIP-1α Ab-treated rats remained higher than those in the control Ab-treated animals (not significant).
Administration of anti-MCP-1 significantly decreased macrophage infiltrations in the ventricular system and the leptomeninges at day 3 and day 7 p.i. Neutralization of MCP-1 resulted in only a slight, nonsignificant reduction of the numbers of migrating granulocytes (Fig. 3 to 5). Furthermore, treatment with neutralizing anti-MCP-1 resulted in down-regulation of MIP-1α, MCP-1, and RANTES mRNA-expressing cells in the brain and elevated MIP-2 mRNA induction at day 7 p.i. (Fig. 6).
Cellular sources of MIP-2, MIP-1α, MCP-1, and RANTES determined by combining in situ hybridization and immunohistochemical staining.
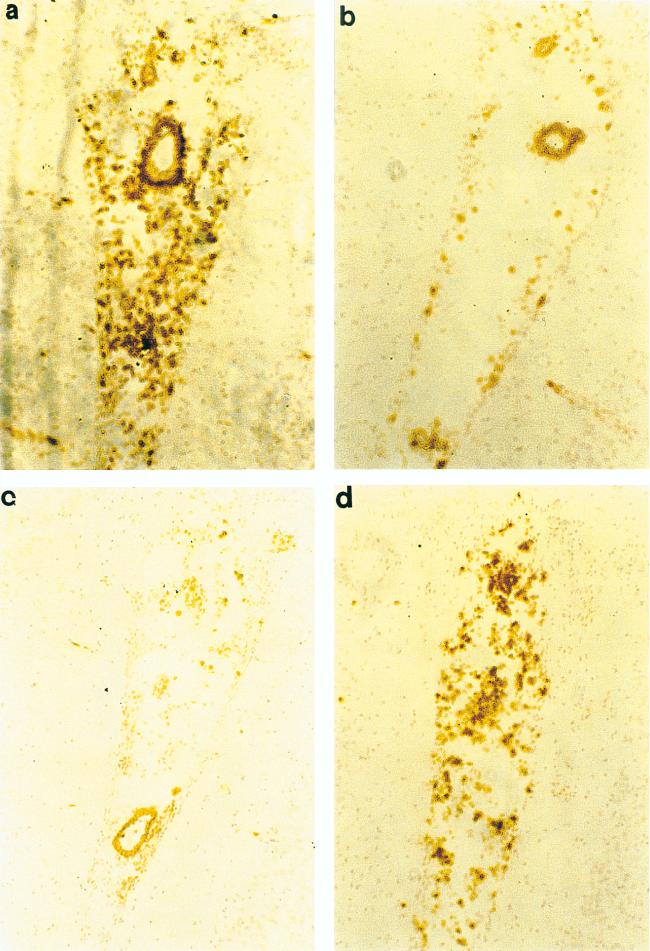
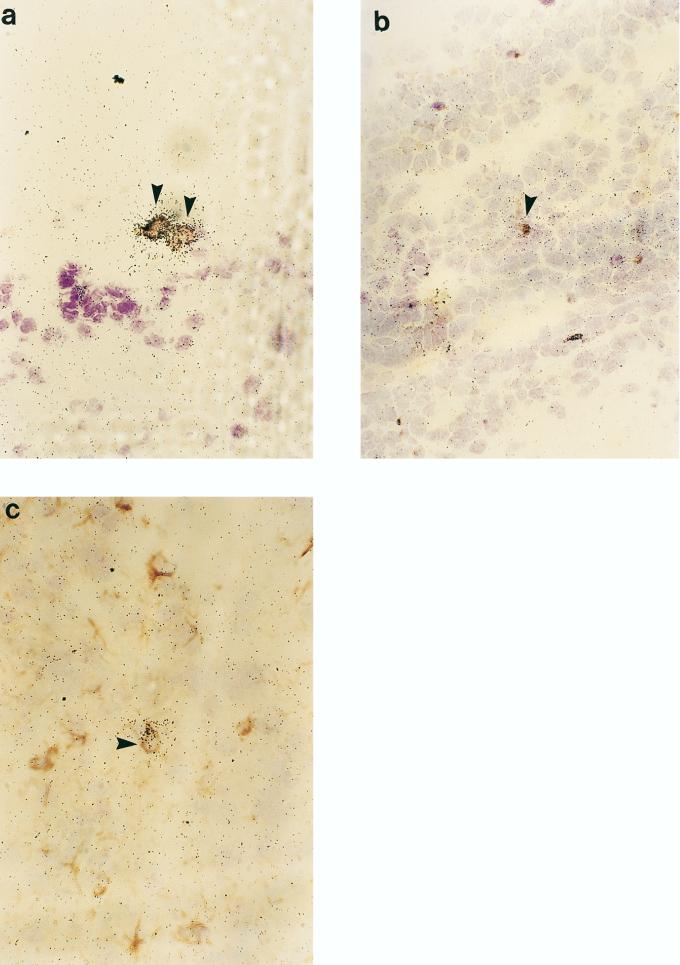
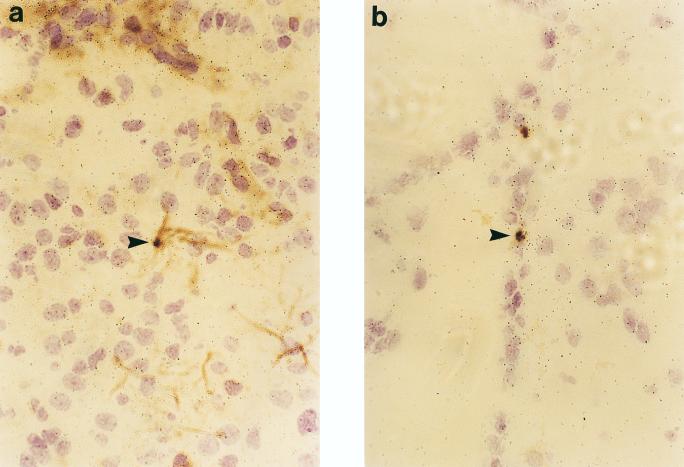
Immunohistochemical stainings for granulocytes, ED1, OX42, CD5, and GFAP were combined with in situ hybridization. At 48 h p.i. the MIP-2- and MIP-1α-positive cells located in the subarachnoid space and the lateral ventricles were granulocytes and macrophages (Fig. 7). It is thus likely that granulocytes and macrophages play a predominant role in MIP-2 and MIP-1α production. No hybridization signals for MIP-2 and MIP-1α were detected in astrocytes or microglia. MCP-1-positive cells were infiltrating granulocytes and macrophages (Fig. 8a and b), and few cells expressing MCP-1 were immunopositive for GFAP, a cellular marker specific to astrocytes (Fig. 8c). RANTES mRNA-positive cells were astrocytes and microglia (Fig. 9). No hybridization signals for any of the chemokines studied were registered in cells positive for CD5, a marker of pan-T cells.
FIG. 7.
Visualization of MIP-2 and MIP-1α mRNA-positive cells in emulsion-dipped brain sections from Hib-infected rats subjected to immunohistochemistry. The photomicrographs of Hib-infected rat brain sections (48 h p.i.) show colocalization of MIP-2 mRNA signals (arrow) with granulocytes (a) and ED1 (b). MIP-1α mRNA-positive cells localize with granulocytes (c) and ED1 (d). Magnification, ×400.
FIG. 8.
Identification of cell types expressing MCP-1 by combining in situ hybridization and immunohistochemistry. Immunohistochemistry was performed for granulocyte (a), ED1 (b), and GFAP (c) determination. Photomicrographs of Hib-infected rat brain sections (48 h p.i.) show MCP-1 mRNA colonization with granulocytes (a), ED1 (b), and astrocytes (c) (arrowheads). Magnification, ×400.
FIG. 9.
Most RANTES mRNA-positive cells were astrocytes (a) (arrowheads and microglia (b). Magnification, ×400.
DISCUSSION
Bacterial meningitis is characterized by the immigration of leukocytes into the subarachnoid space and their subsequent activation. Chemokines have been considered as attractants of leukocytes in bacterial meningitis patients as well as in animal models and were shown to be responsible for the chemoattractant properties of CSF (27, 29, 31). To determine the role of chemokines in eliciting the migration of inflammatory cells to the brain in this model, we first examined their expression during the evolution of the lesion and then established the functional role of likely candidates with blocking agents in vivo.
In the present study, elevated mRNA expression was found for C-X-C chemokine MIP-2 and the C-C chemokines MIP-1α, MCP-1, and RANTES during the early and maximal clinical illness. MIP-2 and MIP-1α mRNA-positive cells were detected during the recovery stage in animals surviving the acute infection (Fig. 2). The absence of clinical symptoms during recovery may also reflect a moderation of chemokine activity by anti-inflammatory cytokines such as IL-10 and transforming growth factor β (8, 11, 14) in the central nervous system. These kinetics were paralleled by those of infiltrating inflammatory cells and disease severity in bacterial meningitis.
The blocking approach enabled us to determine that leukocyte recruitment in response to Hib infection was dependent, at least in part, on production of MIP-2, MIP-1α, and MCP-1, since treatment with neutralizing Abs against each of these chemokines significantly decreased the recruitment of leukocytes into the brain in response to Hib inoculation. Importantly, reduction of leukocyte recruitment was observed to a similar extent at the level of the neutrophil and mononuclear phagocytes irrespective of the Ab used. The data presented in this study showing that blocking with anti-MIP-2 Ab decreases neutrophil accumulation provides further evidence that MIP-2 is an important mediator of neutrophil extravasation and recruitment to extravascular sites. The data are consistent with results obtained from mice deficient in the murine IL-8 receptor homologue (a receptor for MIP-2), in which recruitment of neutrophils into the peritoneal cavity in response to inflammatory stimulation was significantly impaired (4).
In contrast, the observation that Ab to MIP-1α decreases neutrophil recruitment is more difficult to explain, and to our knowledge, it is the first clear description of MIP-1α being implicated in in vivo neutrophil recruitment in Hib-mediated meningitis. A possible explanation for the ability of anti-MIP-1α Ab to reduce neutrophil recruitment is that MIP-1α may contribute indirectly to neutrophil recruitment by enhancing mononuclear phagocyte activation. While previous data have suggested that human MIP-1α recruits and activates neutrophils in the mouse (39), these data are controversial and have not been reproduced in other systems (6, 12, 17). Having observed that neutrophil influx was significantly reduced by anti-MIP-2 and -MIP-1α Abs in vivo, we next determined if blocking MIP-2 and MIP-1α resulted in attenuation of chemokine mRNA expression. Interestingly, blocking with MIP-2 Ab resulted in down-regulation of MIP-2 and RANTES mRNA and up-regulation of MCP-1 mRNA in the brain, while MIP-1α blocking resulted in down-regulation of MIP-1α and RANTES mRNA and up-regulation of MIP-2 and MCP-1 mRNA expression. In addition, anti-MCP-1 Ab resulted in down-regulation of MCP-1, MIP-1α, and RANTES mRNA expression.
The regulatory events controlling the rate of transcription of chemokine genes during the infection process has not yet been studied in detail. Based on our studies, we suspect that the monocyte chemokine MCP-1 plays a significant part in the pathogenesis of bacterial meningitis; it is possible that Ab-mediated blockade fails to affect MCP-1 function, because of its expression in the central nervous system parenchyma. By contrast, MIP-2 and MIP-1α would be more accessible to circulating components, by virtue of their localization in perivascular inflammatory infiltrates. We have shown that neutrophils and monocytes/macrophages are the main sources of MIP-2 and MIP-1α mRNA in the brains of mice with Hib meningitis. Thus, not only macrophages, the well-established source of chemokines, but also neutrophils have the capacity to express the gene for MIP-2 and MIP-1α.
The present data also document the relative role of MCP-1 in mediating the influx of macrophages into the focus of a tissue injury. Unexpectedly, we also detected high MCP-1 and RANTES mRNA levels, although granulocytes were the dominating cell type. Conversely, we noted that anti-MCP-1 Abs significantly attenuated the influx of macrophages in the subarachnoid space. Other not yet characterized factors may also be present and up-regulated in the early stage of Hib meningitis. The contribution of complement factors to the development of CSF pleocytosis has been shown (9). The importance of arachidonic acid pathway metabolites such as leukotrienes and bacterial cell wall products as chemoattractants in bacterial meningitis was shown in rabbits with pneumococcal meningitis (3, 20).
T-lymphocyte recruitment was not observed in the present study despite the fact that MIP-1α, MCP-1, and RANTES mRNA expression was detected in the brain. All these chemokines are chemotactic for human T-lymphocyte subsets in vitro (5, 26, 33), and some evidence for a similar role in vivo exists (18, 19). Finally, direct injection of MIP-1α, MCP-1, and RANTES subcutaneously in mice failed to induce the recruitment of T lymphocytes (34). Taken together, these observations suggest that unlike the case for neutrophils, signals in addition to those provided by a chemotactic factor are required for T-lymphocyte recruitment.
In conclusion, the interplay between chemokines, pathogenic microorganisms, and host inflammatory cells is likely to be a major determinant of the clinical presentation and outcome of Hib meningitis. The further elucidation of the regulation and coordination of chemokine gene expression and chemokine production may lead to novel therapeutic approaches to bacterial meningitis, which at present accounts for substantial morbidity and mortality in humans.
FIG. 4.
Effect of antichemokine Abs on macrophages infiltrating the brains of Hib-infected rats as shown by immunohistochemistry. Shown are representative fields (obtained 72 h p.i.) from animals treated with rabbit Ig as control Ab (a), rabbit polyclonal anti-MIP-2 (b), rabbit polyclonal anti-MIP-1α (c), and rabbit polyclonal anti-MCP-1 (d). (a) Control Ab-treated animals showed diffuse macrophage infiltration in the subarachnoid space. Anti-MIP-2 and anti-MIP-1α-treated animals exhibited less diffuse macrophage infiltration. Animals treated with anti-MCP-1 showed much reduced macrophage infiltration. Staining was performed on 10-μm-thick cryosections as described in Materials and Methods, with immunoperoxidase and hematoxylin. Magnification, ×400.
ACKNOWLEDGMENTS
This study was supported by the Swedish Medical Research Council, the Swedish Association of the Neurologically Disabled (NHR), and funds from the Karolinska Institute.
REFERENCES
- 1.Baggiolini M, Dahinden C A. CC chemokines in allergic inflammation. Immunol Today. 1994;15:127–133. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 2.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greaves D R, Zlotnik A, Schall T J. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 3.Blackham A, Norris A A, Woods F A M. Models for evaluating the anti-inflammatory effects of inhibitors of arachidonic acid metabolism. J Pharm Pharmacol. 1985;37:787–793. doi: 10.1111/j.2042-7158.1985.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 4.Cacalano G, Lee J, Kikly K, Ryan A M, Pitts-Meek S, Hultgren B, Wood W I, Moore M W. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–685. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 5.Carr M W, Roth S J, Luther E, Rose S S, Springer T A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 7.Dagerlind Å, Bean K A J, Hökfelt T. Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry. 1992;98:39–49. doi: 10.1007/BF00716936. [DOI] [PubMed] [Google Scholar]
- 8.Diab A, Zhu J, Lindquist L, Wretlind B, Link H, Bakhiet M. Cytokine mRNA profiles during the course of experimental Haemophilus influenzae bacterial meningitis. Clin Immunol Immunopathol. 1997;85:236–245. doi: 10.1006/clin.1997.4430. [DOI] [PubMed] [Google Scholar]
- 9.Ernst J D, Hartiala K T, Goldstein I M, Sande M A. Complement (C5)-derived chemotactic activity accounts for accumulation of polymorphonuclear leukocytes in cerebrospinal fluid of rabbits with pneumococcal meningitis. Infect Immun. 1984;46:81–86. doi: 10.1128/iai.46.1.81-86.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein G W, Betz A L. The blood-brain barrier. Sci Am. 1986;255:74–83. doi: 10.1038/scientificamerican0986-74. [DOI] [PubMed] [Google Scholar]
- 11.Johns L D, Flanders K C, Ranges G E, Sriram S. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-β1. J Immunol. 1991;147:1792–1796. [PubMed] [Google Scholar]
- 12.Kasama T, Strieter R M, Standiford T J, Burdick M D, Kunkel S L. Expression and regulation of human neutrophil-derived macrophage inflammatory protein-1α. J Exp Med. 1993;178:63–72. doi: 10.1084/jem.178.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelner G S, Kennedy J, Bacon K B, Kleyensteuber S, Largaespada D A, Jenkins N A, Copeland N G, Bazan J F, Moore K W, Schall T J, Zlotnik A. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–1398. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy M K, Torrance D S, Picha K S, Mohler K M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 15.Lawrence M B, Springer T A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 16.Leist T P, Frei K, Kam-Hansen S, Zinkernagel R M, Fontana A. Tumor necrosis factor α in cerebrospinal fluid during bacterial, but not viral, meningitis. Evaluation in murine model infections and in patients. J Exp Med. 1988;167:1743–1748. doi: 10.1084/jem.167.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McColl S R, Hachicha M, Levasseur S, Neote K, Schall T J. Uncoupling of early signal transduction events from effector function in human peripheral blood neutrophils in response to recombinant macrophage inflammatory protein-1α and -1β. J Immunol. 1993;150:4550–4560. [PubMed] [Google Scholar]
- 18.Murphy W J, Taub D D, Anver M, Conlon K, Oppenheim J J, Kelvin D J, Longo D L. Human RANTES induces the migration of human T lymphocyte into the peripheral tissue of mice with severe combined immune deficiency. Eur J Immunol. 1994;24:1823–1827. doi: 10.1002/eji.1830240815. [DOI] [PubMed] [Google Scholar]
- 19.Murphy W J, Tian Z G, Asai O, Funakoshi S, Rotter P, Henry M, Strieter R M, Kunkle S L, Longo D L, Taub D D. Chemokines and T lymphocyte activation. II. Facilitation of human T cell trafficking in severe combined immunodeficiency mice. J Immunol. 1996;156:2104–2111. [PubMed] [Google Scholar]
- 20.Nolan C M, Clark R A, Beaty H N. Experimental pneumococcal meningitis. III. Chemotactic activity in cerebrospinal fluid. Proc Soc Exp Biol Med. 1975;150:134–136. doi: 10.3181/00379727-150-38989. [DOI] [PubMed] [Google Scholar]
- 21.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene ‘intercrine’ cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo J A, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos J C, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 23.Ramilo O, Sáez-Llorens X, Mertsola J, Jafari H, Olsen K D, Hansen E J, Yoshinaga M, Ohkawara S, Nariuchi H, McCracken G H., Jr Tumor necrosis factor α/cachectin and interleukin 1β initiate meningeal inflammation. J Exp Med. 1990;172:497–507. doi: 10.1084/jem.172.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. The role of cytokines in generation of inflammation and tissue damage in experimental Gram-positive meningitis. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schall T J, Bacon K, Toy K J, Goeddel D V. Selective attraction of monocytes and T-lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 26.Schall T J, Bacon K, Camp R D, Kaspare J W, Goeddel D V. Human macrophage inflammatory protein α (MIP-1α) and MIP-1β chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seebach J, Bartholdi D, Frei K, Spanaus K S, Ferrero E, Widmer U, Isenmann S, Strieter R M, Schwab M, Pfister H W, Fontana A. Experimental listeria meningoencephalitis: macrophage inflammatory protein-1α and-2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J Immunol. 1995;155:4367–4375. [PubMed] [Google Scholar]
- 28.Smith A L, Smith D H, Averill D R, Jr, Marino J, Moxon E R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973;8:278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spanaus K S, Nadal D, Pfister H W, Seebach J, Widmer U, Frei K, Gloor S, Fontana A. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J Immunol. 1997;158:1956–1964. [PubMed] [Google Scholar]
- 30.Spellerberg B, Tuomanen E. The pathophysiology of pneumococcal meningitis. Ann Med. 1994;26:411–418. doi: 10.3109/07853899409148362. [DOI] [PubMed] [Google Scholar]
- 31.Sprenger H, Rösler A, Tonn P, Braune H J, Huffmann G, Gemsa D. Chemokines in cerebrospinal fluid of patients with meningitis. Clin Immunol Immunopathol. 1996;80:155–161. doi: 10.1006/clin.1996.0109. [DOI] [PubMed] [Google Scholar]
- 32.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–322. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 33.Taub D D, Conlon K, Lloyd A R, Oppenheim J J, Kelvin D J. Preferential migration of activated CD4+ and CD8+ T-cells in response to MIP-1α and MIP-1β. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 34.Tessier P A, Naccache P H, Clark-Lewis I, Gladue R P, Neote K S, McColl R. Chemokine network in vivo. Involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-α. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 35.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 36.Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor α, interleukin-1, and interleukin-6 in meningococcal meningitis. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe K, Iida M, Takaishi K, Suzuki T, Hamada Y, Iizuka Y, Tsurufuji S. Chemoattractants for neutrophils in lipopolysaccharide-induced inflammatory exudate from rats are not interleukin-8 counterparts but gro-gene-product/melanoma-growth-stimulating-activity-related factors. Eur J Biochem. 1993;214:267–270. doi: 10.1111/j.1432-1033.1993.tb17920.x. [DOI] [PubMed] [Google Scholar]
- 38.Wolpe S D, Cerami A. Macrophage inflammatory protein 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989;3:2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- 39.Wolpe S D, Davatelis G, Sherry B, Beutler B, Hesse D G, Nguyen T, Moldaver L L, Nathan C F, Lowry S F, Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;167:570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolpe S D, Sherry B, Juers D, Davatelis G, Yurt R W, Cerami A. Identification and characterization of macrophage inflammatory protein-2. Proc Natl Acad Sci USA. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]