Abstract
Background
Recurrent pneumonia (RP) accounts for 7.7%–9% of childhood pneumonia. Identifying the cause of RP is crucial for infection control and reducing mortality and morbidity. This study aimed to investigate the etiology, risk factors, and diagnostic procedures of RP based on the literature using a diagnostic algorithm.
Methods
Our study included RP patients aged 0–18 years who were followed up as outpatients or inpatients between 2018 and 2021. Patients were analyzed retrospectively using the national health database. Patients with RP were identified by ICD diagnosis codes. Etiology and risk factors were determined based on the occurrence of RP in the same or different areas.
Results
The rate of RP was found to be 14.4% among the cases of recurrent lower respiratory tract infection. Of these, 27.6% occurred in the same area and 72.4% in different areas. The underlying etiology was identified in 224 of 250 RP patients (89.6%). RP in different areas was mainly due to neuromuscular diseases, whereas asthma, right middle lobe syndrome, and congenital lung or airway structural disease were common causes of RP in the same area. Malnutrition, passive smoke exposure, and prematurity were common risk factors in both groups.
Conclusions
Systematic etiological investigations should take into account the characteristics of the patient population and geographical region. The use of diagnostic algorithms based on recurrence in the same or different areas is particularly beneficial. Whether asthma is causally related to RP episodes or is a coincidental association due to inadequate differential diagnosis remains unclear.
Keywords: child, diagnosis, pneumonia, reinfection, risk factors
1. INTRODUCTION
Community‐acquired pneumonia continues to be an important cause of morbidity and mortality in children worldwide and in our country. Pneumonia is diagnosed in 23% of all pediatric outpatients and 33%–50% of hospitalized children aged 0–1 year. Approximately 6% of infants have at least one episode of pneumonia in the first 2 years of life. 1 Recurrent pneumonia (RP) accounts for 7.7%–9% of pneumonia in childhood. 2 , 3 RP is defined as at least two episodes of pneumonia in 1 year or at least three episodes in any period and radiological improvement between episodes. 4 , 5 RP is a common cause of presentation in general pediatric clinics, and untreated patients are frequently referred to pediatric pulmonology clinics.
The challenge for RP is to distinguish between children with self‐limiting illnesses that do not require further diagnostic work‐up and children with an underlying disease that requires further investigation. Healthy preschool children aged 2–4 years are at risk of pneumonia, especially in autumn and winter when there are many respiratory viruses and pneumonia may recur. 6 , 7 , 8 Preschool children whose infections resolve spontaneously, who have no complaints between attacks, whose other characteristics such as growth and development, are not affected, and who have pneumonia in winter rather than summer usually do not require further investigation. Those with more severe forms of pneumonia at an earlier age should be questioned about any potential risk factors and comorbid conditions. 6 , 7
Once a patient requiring advanced diagnostic techniques has been identified, the next step is to determine the localization of the RP. The use of specific algorithms for the diagnostic approach to RP helps to prevent unnecessary investigations and waste of time and money and offers the opportunity for faster diagnosis and treatment. It is important to note that diagnostic tests and facilities may differ depending on the country, region, and health center, which may lead to different etiological causes being detected differently. Since 2018, RP has been analyzed in two groups: RP occurring in the same area and RP that affects different areas of the lung in our clinic. “The algorithms of the diagnostic approach to RP” by Montella et al. 6 were used according to the results of a meta‐analysis, with modifications made according to the facilities and characteristics of our country and our center. The objective of this study was to discuss the frequency of RP, its etiological causes, risk factors, and our diagnostic approach in our general pediatric services, including the pediatric pulmonology department, in light of the literature.
2. MATERIALS AND METHODS
Between 1 January 2018 and 31 December 2021, patients aged 0–18 years with a diagnosis of lower respiratory tract infection (LRTI) and an ICD code requiring admission to the departments of Pediatric Pulmonology and General Pediatrics, who were followed up as outpatients or inpatients, were retrospectively included in the study.
Patients diagnosed with LRTI were retrospectively screened through the national health database, the online database of our hospital and patient files, and recurrent LRTI patients were identified. If any one or more of the ICD codes of the specified LRTI diagnosis was found twice in 1 year or at least three times in any period (during the 4‐year study period), it was considered as “Recurrent LRTI” (Figure 1).
Figure 1.

Identification of recurrent pneumonia patients and ICD codes for lower respiratory tract infection.
The patients with recurrent LRTI, current demographic characteristics of the patients (gender, age, height, and weight), age at the time of first admission to our center for pneumonia, age at first episode of pneumonia, total number of pneumonia episodes, total number of hospitalizations were recorded. The age at first episode of pneumonia and the total number of pneumonia episodes were determined by searching the national health database for the health records of patients before their first admission to our center for pneumonia. “Age at first admission to our center for pneumonia” and “age at first episode of pneumonia” were further subdivided into “1–6 months,” “7–12 months,” “13–36 months,” “37–60 months,” and “≥61 months”. The presence of predicted risk factors for RP and associated clinical findings were recorded. Risk factors have been identified based on extensive research about this subject and textbook information. Routine laboratory tests, imaging results, and specific tests of some disease groups thought to be responsible for the etiology of RP were recorded and investigated.
The presence of cough, chest retractions, tachypnea, and fever were considered as “suspected pneumonia.” Chest radiographs and computed tomography (CT) scans of these patients registered in the online system were analyzed. Those whose diagnosis of pneumonia was confirmed by chest X‐ray or CT scan were considered as having “pneumonia.” The patients who had “two episodes of pneumonia within a year or at least three episodes of pneumonia in any period, provided that there was at least 1 month of radiological improvement between pneumonia episodes,” were considered as having RP.
The patients were divided into two groups, “RP in the same area” and “RP in different areas,” according to the anatomical localization of RP in the lung. For the etiological investigation, two diagnostic algorithms were modified according to our country, our patient population, and the diagnostic facilities of the hospital to clarify these two groups (Figure 2).
Figure 2.
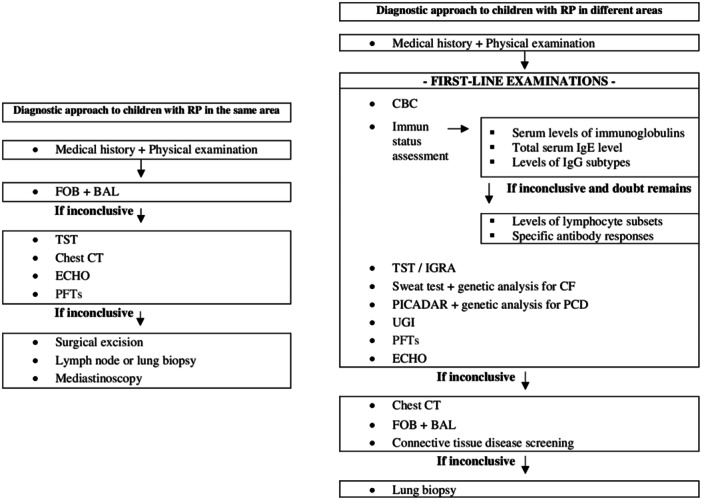
Diagnostic algorithm according to the area of recurrent pneumonia in children. BAL, bronchoalveolar lavage; CBC, complete blood count; CF, cystic fibrosis; CT, computed tomography; ECHO, echocardiography; FOB, fiberoptic bronchoscopy; IGRA, interferon gamma releasing assay; PCD, primary ciliary dyskinesia; PFTs, pulmonary function tests; PICADAR, primary ciliary dyskinesia rule; TST, tuberculin skin test; UGI, upper gastrointestinal investigations.
The study was approved by Istanbul Medeniyet University Göztepe Prof. Dr. Süleyman Yalçın City Hospital Ethics Committee (date: 12.01.2022, number 2021/0710).
Data analysis was performed using the SPSS 25.0 package program. Recurrent pneumonias were compared using the Chi‐squared test according to categories such as location (same or different areas), etiology, demographics, risk factors, attack groups, and findings indicating the need for further investigation. For the Chi‐squared test, we used the likelihood ratio p‐value if there were fewer than five observations in a group. We compared recurrent pneumonia in the same or different areas using numerical parameters by independent sample t‐test. The level of statistical significance (α = .05) was accepted for all comparisons.
3. RESULTS
Of the 13,387 patients registered with ICD codes for LRTI over a 4‐year period, 1735 (12.9%) were found to have recurrent LRTI. Of the 1735 patients with recurrent LRTI, 14.4% (n = 250) met the criteria for RP. Of RP patients, 224 (89.6%) had an underlying etiology.
3.1. Demographic characteristics
The M/F ratio was found to be 1.08 (130/120).
3.1.1. Age at the time of first admission to our center for pneumonia
The mean age was 48.18 ± 44.60 (1–192) months. The number of patients aged 61 months and older (30.4%) was significantly higher at first admission (p < .005). The second most common age group was 13–36 months (23.2%), followed by 37–60 months (19.6%).
3.1.2. Age at first episode of pneumonia
The mean age was 37.84 ± 39.83 (1–187) months.
Among our patients, 25.2% had their first pneumonia after 61 months. The second most common age group for the first episode of pneumonia was 1–6 months, with a rate of 22.8%. Of these, 22.8% had a diagnosis of NMD, and 21% had a diagnosis of congenital lung or airway structural diseases. Among patients who had RP in different areas, the first pneumonia episode of RP was most common between the ages of 1–12 months and 61 months and older. In patients with RP in the same area, the first pneumonia episode was more common between 12 and 61 months (p: .001).
Analyzing etiologies by age at first pneumonia, 77.8% of GERD patients, 59.2% of congenital lung or airway structural disease patients, 50% of congenital heart disease (CHD) patients, 43% of NMD patients, and 41.7% of primary immune deficiency (PID) patients had their first pneumonia episode before the age of 1 year.
3.2. Data of recurrent pneumonia (RP)
In our RP patients, neuromuscular diseases (NMD) were the most frequent etiologies (20.4%), followed by asthma (19.2%) and congenital lung or airway structural diseases (10.8%) (Table 1). Of the cases of RP, 27.6% were in the same area and 72.4% were in different areas.
Table 1.
Etiologies of RP patients.
| n (%) | n (%) | n (%) | p | |
|---|---|---|---|---|
| Total 250 (100) | Same area 69 (27.6) | Different areas 181 (72.4) | ||
| Neuromuscular diseases | 51 (20.4) | 3 (4.3) | 48 (26.5) | .000 |
| Asthma | 48 (19.2) | 20 (29) | 28 (15.5) | .015 |
| Congenital lung or airway structural diseases | 27 (10.8) | 15 (21.7) | 12 (6.6) | .001 |
| Right middle lobe syndrome | 18 (7.2) | 18 (26.1) | 0 (0) | .000 |
| Congenital heart diseases | 14 (5.6) | 5 (7.2) | 9 (5) | .485 |
| Cystic fibrosis | 14 (5.6) | 0 (0) | 14 (7.7) | .017 |
| Primary immune deficiencies | 12 (4.8) | 1 (1.4) | 11 (6.1) | .126 |
| Bronchiectasis | 12 (4.8) | 2 (2.9) | 10 (5.5) | .385 |
| Primary ciliary dyskinesia | 11 (4.4) | 0 (0) | 11 (6.1) | .059 |
| Gastroesophageal reflux disease | 9 (3.6) | 0 (0) | 9 (5) | .036 |
| Interstitial lung diseases | 6 (2.4) | 1 (1.4) | 5 (2.8) | .544 |
| Foreign body aspiration | 2 (0.8) | 2 (2.9) | 0 (0) | .021 |
| Unknown | 26 (10.4) | 2 (2.9) | 24 (13.3) | .016 |
3.2.1. The number of episodes for recurrent pneumonia
The mean total number of pneumonia episodes in all patients was 4.10 ± 2.46 (2–18). The mean number of episodes was significantly lower in the patients who had RP in the same area (3.20 ± 1.587) compared to the patients who had RP in different areas (4.4 ± 2.646) (p: .000). The majority of our patients (46.4%) were diagnosed with RP and had more than three episodes. The etiology was detected at the third episode and later in 20.4% of patients with RP in different areas, and at the second episode in 40.3% of patients with RP in the same area (p: .000) (Table 2).
Table 2.
Departmental data of RP patients.
| n (%) | Total | Same area | Different areas | p | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| Sections for RP 250 (100) | 2 episodes in a year | 63 (25.2) | 28 (40.6) | 35 (19.3) | .000 |
| 3 episodes in any period | 71 (28.4) | 23 (33.3) | 48 (26.5) | ||
| >3 episodes in any period | 116 (46.4) | 18 (26.1) | 98 (54.1) | ||
| Diagnostic section 224 (89.6) | Before the first episode | 103 (46) | 24 (35.8) | 79 (50.3) | .046 |
| After the first episode | 121 (54) | 43 (64.2) | 78 (49.7) | ||
| Diagnostic section 224 (89.6) | Before the first episode | 103 (46) | 24 (35.8) | 79 (50.3) | .000 |
| First episode | 30 (13.4) | 7 (10.4) | 23 (14.6) | ||
| Second episode | 50 (22.3) | 27 (40.3) | 23 (14.6) | ||
| Third episode and following | 41 (18.3) | 9 (13.4) | 32 (20.4) | ||
Abbreviation: RP, recurrent pneumonia.
The results showed significant reductions in episode frequency of 26.9%, 12.7%, and 16.4% in patients with asthma, right middle lobe syndrome, and unknown etiology, respectively.
3.2.2. Associated clinical findings for recurrent pneumonia
The most common associated clinical findings were growth and developmental retardation (34%), wheezing (31.6%), productive or wet cough (25.2%), chest wall deformities (11.2%), clubbing (2.8%), and accompanying neurological problems (21.2%).
3.2.3. Risk factors for recurrent pneumonia
Among our RP patients, malnutrition (43.6%), passive smoke exposure (31.6%), prematurity (23.2%), and cerebral palsy (16.8%) were the most frequently identified risk factors (Table 3).
Table 3.
Predicted and evaluated risk factors in RP patients.
| Total | Same area | Different areas | p | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Prematurity | 58 (23.2) | 12 (17.4) | 46 (25.4) | .179 |
| Bronchopulmonary dysplasia | 19 (7.6) | 5 (7.2) | 14 (7.7) | .896 |
| Cerebral palsy | 42 (16.8) | 5 (7.2) | 37 (20.4) | .013 |
| Congenital heart diseases | 20 (8) | 6 (8.7) | 14 (7.7) | .802 |
| Atopy | 37 (14.8) | 14 (20.3) | 23 (12.7) | .131 |
| Malnutrition (Acute) | 36 (14.4) | 10 (14.5) | 26 (14.4) | .790 |
| Malnutrition (Chronic) | 73 (29.2) | 18 (26.1) | 55 (30.4) | |
| Passive smoke exposure | 79 (31.6) | 21 (30.4) | 58 (32) | .807 |
| PEG | 37 (14.8) | 5 (7.2) | 32 (17.7) | .038 |
| Tracheostomy | 14 (5.6) | 1 (1.4) | 13 (7.2) | .078 |
Abbreviations: PEG, percutaneous endoscopic gastrostomy; RP, recurrent pneumonia.
In different areas, risk factors such as cerebral palsy and the presence of PEG were more common (p: .013 and .038, respectively). Patients with “cerebral palsy, malnutrition, PEG, tracheostomy and BPD” had significantly more hospitalizations (p: .000/.000/.000/.000/.000/.000/.000/.000/.021, respectively). It was found significant that 43.3% of the patients with cerebral palsy had seven or more episodes (p: .000). Four‐six episodes occurred in 54.7% of the patients who had malnutrition and in 15.1% of the patients who had CHD (p: .021/p: .010, respectively). It was significant that seven or more episodes occurred in 46.7% and 23.3% of the patients with PEG and tracheostomy, respectively (p: .000).
3.2.4. Fiber optic bronchoscopy (FOB) findings
FOB and bronchoalveolar lavage (BAL) culture were performed in 30% of patients. Only 22.7% of patients had growth in the BAL culture. Of these, 35.3% were Haemophilus influenzae, 29.4% were Moraxella catarrhalis, 17.6% were Pseudomonas aeruginosa and 5.9% each were Staphylococcus aureus, Candida albicans and Streptococcus pneumoniae. Airway obstruction due to external compression was found significantly more often when RP occurred in the same area (p: .01).
3.2.5. Prognosis
The mortality rate of our patients was found to be 8%. Of these patients, 90% had NMD, and 75% had growth and developmental retardation.
4. DISCUSSION
The prevalence of RP in patients with LRTI was 14.4%, of which 27.6% were in the same area, and 72.4% were in different areas. The underlying cause of RP was identified in 89.6%. The most common cause of RP in different areas is NMD, whereas the most common causes of RP in the same area are asthma, right middle lobe syndrome, and congenital lung or airway structural diseases. Patients with RP from different areas presented at an earlier age exhibited a higher frequency of pneumonia recurrence and were hospitalized more frequently. However, the most common risk factors, including malnutrition, passive smoke exposure, and prematurity, were similar in both groups. The mortality rate in our RP patients was found to be 8%, and the largest group was constituted by the patients who had NMD with a rate of 90%.
Previous studies have shown that RP accounts for 7.7%–9% of all pneumonias. 2 , 3 As a result of the retrospective nature of our study, the rate of RP in the recurrent LRTI population according to ICD diagnosis codes was found to be 14.4%. All file data of patients with recurrent LRTI were reviewed and included in our analysis after the diagnosis of pneumonia was confirmed clinically and radiologically.
It was not possible to identify the cause in all patients in any of the studies aimed to identify the etiology of RP, and the percentage of identifying the etiology varied between 69.4% and 96.4%. 9 , 10 In our study, the etiology could not be identified in 10.4% of the patients. This rate was reported to be 30.6% in the study conducted by Hoving et al., 9 but asthma was not considered a cause in this study unlike other studies. It is our contention that the patients whose etiology could not be determined were children with self‐limiting or minor problems that did not require further diagnostic work‐up. This is evidenced by the fact that 84.6% of these patients had at most two or three episodes.
The most common etiologies were identified: NMD, asthma, congenital lung or airway structural diseases, and right middle lobe syndrome. The rates and differences in etiological factors in various studies are shown in Table 4. Diagnostic approaches, clinician experiences, differences in patient population, and geographical location may explain the variability in the rates of the etiologies of RP. 6 , 11 , 12 , 13
Table 4.
Causes of RP in the current study compared with previous studies.
| Variable | Current Study | Owayed et al. 2 | Lodha et al. 3 | Montella et al. 6 | Hoving et al. 9 | Çiftçi et al. 11 | Patria et al. 15 ** |
|---|---|---|---|---|---|---|---|
| Country | Türkiye | Canada | India | Italy | The Netherlands | Türkiye | Italy |
| Adjustment | Tertiary care center; Pediatric pulmonology clinic | Tertiary care center; General pediatric clinic | Tertiary care center; Pediatric pulmonology clinic | Tertiary care center; Pediatric pulmonology clinic | General hospital; General pediatric clinic | Tertiary care center; Pediatric infectious diseases clinic | Tertiary care center; Pediatric pulmonology clinic |
| Number of patients | 250 | 238 | 70 | 113 | 62 | 71 | 146 |
| Diagnosis rate (%) | 90 | 92 | 84 | 83 | 69 | 84 | NA |
| Underlying causes (%) | |||||||
| NMD | 20 | NA | NA | NA | NA | NA | NA |
| Asthma | 19 | 8 | 14 | NA | NA | 32 | 31 |
| Congenital lung or airway structural diseases | 11 | 8 | 9 | 11 | 16 | 6 | NA |
| Right middle lobe syndrome | 7 | NA | NA | 15 | NA | NA | 30 |
| Congenital heart diseases | 6 | 9 | 3 | 2 | 5 | 8 | 2 |
| Cystic fibrosis | 6 | NA | NA | 4 | NA | 3 | NA |
| PID | 5 | 14 | 16 | 7 | 16 | 10 | 1 |
| PCD | 4 | NA | 7 | 38 | NA | NA | 1 |
| Recurrent aspiration/GERD * | 4 | 53 | 21 | 5 | 26 | 18 | 24 |
Abbreviations: GERD, gastroesophageal reflux disease; NMD, neuromuscular diseases; PCD, primary ciliary dyskinesia; PID, primary immune deficiencies; RP, recurrent pneumonia.
Only isolated GERD patients were included in our study. Others were includes all aspiration syndromes including NMD.
More than one factor was determined for one patient.
In the majority of studies, aspiration syndromes are regarded as one of the most prevalent causes of RP. In some studies in the literature, NMD was evaluated within the scope of aspiration syndromes. 2 , 14 , 15 However, aspirations alone are not responsible for RP in NMD. NMD may involve most respiratory muscle groups including inspiratory, expiratory and bulbar muscles. The natural course of NMD is characterized by ineffective cough and swallowing disorders leading to chronic aspiration, poor secretion clearance, pneumonia and hypercapnic respiratory failure. 16 , 17 , 18 It is more logical to consider NMD as a separate group. Aspiration‐independent mechanisms also contribute to the development of pneumonia. Our relatively lower rate of recurrent aspiration compared to different studies was associated with the NMD group which was not included in the recurrent aspiration group (Table 4).
Although asthma was found to be the second most common cause of RP in our study, there are still many question marks in the literature on this subject. These may be cases in which we could not identify the exact cause or cases with concomitant asthma. Asthma is not often associated with RP, and it is not always clear whether their co‐occurrence in children represents a potential etiological relationship or whether they coincidentally overlap. 19 However, asthma has been implicated as an etiological factor in several studies to date. Only in the study conducted by Hoving et al., 9 asthma was never diagnosed as an underlying cause. Baseer et al. 14 considered it as a predisposing factor. Tachypnea and cough are common symptoms of both asthma and pneumonia. “Asthma‐like” respiratory symptoms are extremely common and require careful differential diagnosis of asthma, especially in children under 5 years. In addition, pneumonia is not mentioned among the complications of asthma in asthma guidelines. 20 In studies with data obtained from retrospective registries such as ours, adherence to the data is mandatory and a better differential diagnosis is not possible.
In clinical practice, right middle lobe syndrome is a common cause of RP occurring in the same area. 21 There is no collateral ventilation between the middle lobe and other lobes, which reduces the likelihood of re‐expansion after atelectasis develops. In our study, 7.2% of the patients had right middle lobe syndrome in the etiology. Montella et al. 6 reported this rate to be 15%, while Patria et al. 15 reported a rate of 30.1%. The lower rate of right middle lobe syndrome compared to other studies may be related to the fact that patients with both right middle lobe syndrome and asthma were included in the asthma group.
To determine the etiology of RP with the greatest accuracy, rapidity, and minimal examination, it is important to consider the approach according to the recurrence area. Similar to the results of our study, Montella et al. 6 reported that 24.8% of their patients had RP occurring in the same area, and 75.2% had RP occurring in different areas. Hoang et al. 22 reported that 18.2% of their patients had RP occurring in the same area and 81.8% had RP occurring in different areas. Therefore, we estimate that the ratio of RP occurring in different areas to RP occurring in the same area is approximately 3/1.
Similar to our study, the only data in the literature that distinguished RP according to the areas of recurrence and compared the etiologies accordingly belong to Montella et al. 6 Right middle lobe syndrome, structural lung, and airway diseases were frequently found in cases of RP occurring in the same area. Aspirations, inherited lung diseases such as CF and PCD, and immunodeficiencies were predominant in cases of RP occurring in different areas. Compared with the present study, the low rate of the diagnosis of PCD may be explained by the lack of widespread use of advanced diagnostic methods for PCD (video fluoroscopy and electron microscopy) and difficulty in accessing these diagnostic methods in our center and in our country.
Before their first episode, about half of our patients had a known etiology. This is particularly important as NMD constitutes the largest group in our patient population. Çiftçi et al. 11 reported that only 18.3% of patients had knowledge of the etiology before the first pneumonia episode. This study suggests that asthma is the most common cause of the condition and that patients are only diagnosed after experiencing recurrent episodes. Cabezuelo et al. 23 diagnosed 72% of patients during the first pneumonia episode, which may be related to the high incidence of CHD and early diagnosis with different presentations. These data show that there is a direct correlation between the time of identification of the etiology and the most commonly identified etiological factors.
It is not recommended that each child, who meets the definition of RP, should be investigated comprehensively, and it is emphasized that the economic burden should be taken into consideration. 6 However, in our patients, if there are frequent associated clinical findings such as growth and developmental retardation, wheezing, productive or wet cough, chest wall deformities, clubbing and accompanying neurological problems, more careful investigation of the etiology may be considered. The most common associated clinical findings were growth and developmental retardation and wheezing. Considering that our two largest etiological groups were NMD and asthma, we can say that this result is not coincidental.
Identification of risk factors for RP will facilitate the diagnosis and treatment approach. 6 Malnutrition and passive smoke exposure are significant risk factors for the development and poor prognosis of pneumonia. 24 , 25 , 26 , 27 In our study, malnutrition and passive smoke exposure were the most prevalent risk factors. Montella et al. 6 reported passive smoke exposure with a rate of 31.8%, atopy with a rate of 26.5%, and prematurity with a rate of 11.5%; Baseer et al. 14 reported malnutrition with a rate of 63.2%, passive smoke exposure with a rate of 42.5%, prematurity with a rate of 32.1%, CHD with a rate of 27.6%, and atopy with a rate of 23%.
Bronchoscopy is an important investigation method, especially for RP occurring in the same area. Although it was performed in 30% of all our patients, the rate was quite low. This might be related with the facts that 55% of the patients who had RP in the same area had asthma and right middle lobe syndrome, had fewer episodes, were hospitalized less frequently and did not require further investigation. “Bronchial narrowing due to external compression” was observed more frequently in cases of RP occurring in the same area compared to the cases of RP occurring in different areas.
Patients with any of the following risk factors were found to be more likely to be hospitalized: cerebral palsy, malnutrition, presence of PEG, presence of tracheostomy, and BPD. Patients with “PEG, tracheostomy, and cerebral palsy” had an average of seven or more episodes due to their existing multiple problems. The higher number of hospitalizations and episodes in patients with PEG was attributed to the underlying cause being NMD in most of these patients. It should be kept in mind that PEG alone cannot be responsible for the number of hospitalizations and episodes. The same logic applies to the increased number of episodes in patients with tracheostomy.
Our mortality rate was found to be 8%, and most of these patients had NMD, and 75% had growth and developmental retardation in the etiology. In another study from our country, 28 the exitus rate was reported to be 9%, and most of these patients had neuromental retardation in the etiology. The presence of multiple comorbid conditions in patients in the NMD spectrum may be associated with a high mortality rate.
Our study has some limitations. The high rate of malnutrition and NMD makes the sample less homogeneous compared to other studies. Since the study was retrospective, the source of our patient population was ICD codes. It must be noted that these ICD codes may not always accurately reflect the patient's clinical presentation. In addition, we did not have the chance to confirm the diagnosis of asthma diagnosed with recurrent clinical findings, especially in children under 5 years of age, and this may be an over diagnosis.
5. CONCLUSION
In RP patients, etiological investigations should be systematic and take into account the characteristics of the patient population and geographical region. The utilization of diagnostic algorithms according to recurrence in the same or different areas is a particularly beneficial aspect of this process.
It is unclear whether there is a causal relationship between asthma and RP episodes or whether it is a coincidental association due to inadequate differential diagnosis. In this context, we believe that prospective studies applying diagnostic algorithms may be more comprehensive and informative.
AUTHOR CONTRIBUTIONS
Taha Özçelik: Conceptualization; investigation; writing—original draft; methodology; validation; visualization; writing—review & editing; project administration; formal analysis; data curation; resources; funding acquisition; supervision; software. Sinem Can Oksay: Conceptualization; investigation; writing—original draft; methodology; validation; visualization; writing—review & editing; software; formal analysis; project administration; data curation; supervision. Saniye Girit: Conceptualization; investigation; writing—original draft; methodology; validation; writing—review & editing; formal analysis; visualization; software; project administration; supervision; data curation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The original article was written in accordance with the Declaration of Helsinki. The study was approved by Istanbul Medeniyet University Göztepe Prof. Dr. Süleyman Yalçın City Hospital Ethics Committee (date: 12.01.2022, number 2021/0710).
ACKNOWLEDGMENTS
We acknowledge that we employed ChatGPT 3.5 and 4 to assist us in refining the clarity of our writing while developing the draft of this original article. We always maintained continuous human oversight (editing‐revising) and verified the artificial intelligence‐generated output. We never used AI to find, locate, or review the literature or resources, summarize the articles, analyze the selected articles, or synthesize the findings. The authors completed all analyses with higher‐level efforts. The authors declare that the study received no funding.
Özçelik T, Can Oksay S, Girit S. Diagnostic approach to the etiology of recurrent pneumonia in children. Pediatr Pulmonol. 2024;59:3650‐3659. 10.1002/ppul.27275
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Sema U, Sevgi BS. Turkish Thoracic Society consensus report on the diagnosis and treatment of community‐acquired pneumonia in children. Turk Thorac J. 2009;10:10‐13. [Google Scholar]
- 2. Owayed AF, Campbell DM, Wang EEL. Underlying causes of recurrent pneumonia in children. Arch Pediatr Adolesc Med. 2000;154(2):190‐194. 10.1001/ARCHPEDI.154.2.190 [DOI] [PubMed] [Google Scholar]
- 3. Lodha R, Puranik M, Natchu UCM, Kabra SK. Recurrent pneumonia in children: clinical profile and underlying causes. Acta Paediatr. 2002;91(11):1170‐1173. 10.1080/080352502320777388 [DOI] [PubMed] [Google Scholar]
- 4. de Benedictis FM, Bush A. Recurrent lower respiratory tract infections in children. BMJ. 2018;362:k2698. 10.1136/BMJ.K2698 [DOI] [PubMed] [Google Scholar]
- 5. Wald ER. Recurrent and unresolved pneumonia in children. Semin Respir Infect. 1993;8(1):46‐58. [PubMed] [Google Scholar]
- 6. Montella S, Corcione A, Santamaria F. Recurrent pneumonia in children: a rational diagnostic approach and single centre experience. Int J Mol Sci. 2017;18(2):296. 10.3390/IJMS18020296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patria MF, Esposito S. Recurrent lower respiratory tract infections in children: a practical approach to diagnosis. Paediatr Respir Rev. 2013;14(1):53‐60. 10.1016/J.PRRV.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 8. Ostapchuk M, Roberts DM, Haddy R. Community‐acquired pneumonia in infants and children. Am Fam Physician. 2004;70(5):899‐908. [PubMed] [Google Scholar]
- 9. Hoving MP, Brand PL. Causes of recurrent pneumonia in children in a general hospital. J Paediatr Child Health. 2013;49(3):E208‐E212. 10.1111/jpc.12114 [DOI] [PubMed] [Google Scholar]
- 10. Barakat AN, Hussein MM, Fouda EM, Zoair AM, El‐Razek AMA. Underlying causes of recurrent pneumonia in children: a two‐centre study. J Adv Med Med Res. 2021;33:62‐69. 10.9734/jammr/2021/v33i630861 [DOI] [Google Scholar]
- 11. Çiftçi E, Güneş M, Köksal Y, İnce E, Doġru Ü. Underlying causes of recurrent pneumonia in Turkish children in a university hospital. J Trop Pediatr. 2003;49(4):212‐215. 10.1093/TROPEJ/49.4.212 [DOI] [PubMed] [Google Scholar]
- 12. Groves M, O'Rourke P, Alexander H. Clinical reasoning characteristics of diagnosticians. Med Teach. 2003;25(3):308‐313. 10.1080/0142159031000100427 [DOI] [PubMed] [Google Scholar]
- 13. Norman G, Young M, Brooks L. Non‐analytic models of clinical reasoning: the role of experience. Med Educ. 2007;41:1140‐1145. 10.1111/j.1365-2923.2007.02914.x [DOI] [PubMed] [Google Scholar]
- 14. Abdel Baseer KA, Sakhr H. Clinical profile and risk factors of recurrent pneumonia in children in Qena governorate, Egypt. Int J Clin Pract. 2021;75(4):e13695. 10.1111/ijcp.13695 [DOI] [PubMed] [Google Scholar]
- 15. Patria F, Longhi B, Tagliabue C, et al. Clinical profile of recurrent community‐acquired pneumonia in children. BMC Pulm Med. 2013;13(1):60. 10.1186/1471-2466-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Millman AJ, Finelli L, Bramley AM, et al. Hospitalisation for community‐acquired pneumonia in children with neurological impairments. J Pediatr. 2016;173:188‐195.e4. 10.1016/j.jpeds.2016.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cherchi C, Chiarini Testa MB, Deriu D, et al. All you need is evidence: what we know about pneumonia in children with neuromuscular diseases. Front Pediatr. 2021;9:625751. 10.3389/fped.2021.625751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Healy F, Panitch HB. Pulmonary complications of pediatric neurological disorders. Pediatr Ann. 2010;39(4):216‐224. 10.3928/00904481-20100318-06 [DOI] [PubMed] [Google Scholar]
- 19. Heffelfinger JD, Davis TE, Gebrian B, Bordeau R, Schwartz B, Dowell SF. Evaluation of children with recurrent pneumonia diagnosed according to World Health Organisation criteria. Pediatr Infect Dis J. 2002;21(2):108‐112. 10.1097/00006454-200202000-00005 [DOI] [PubMed] [Google Scholar]
- 20. Venkatesan P. 2023 GINA report for asthma. Lancet Respir Med. 2023;11(7):589. 10.1016/S2213-2600(23)00230-8 [DOI] [PubMed] [Google Scholar]
- 21. Panitch HB. Evaluation of recurrent pneumonia. Pediatr Infect Dis J. 2005;24(3):265‐266. 10.1097/01.INF.0000156419.60574.16 [DOI] [PubMed] [Google Scholar]
- 22. Hoang KL, Ta AT, Pham VT. Severe recurrent pneumonia in children: underlying causes and clinical profile in Vietnam. Ann Med Surg. 2021;67:102476. 10.1016/j.amsu.2021.102476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cabezuelo Huerta G, Vidal Micó S, Abeledo Gómez A, Frontera Izquierdo P. Causas subyacentes de neumonía recurrente. An Pediatr. 2005;63(5):409‐412. 10.1157/13080405 [DOI] [PubMed] [Google Scholar]
- 24. Saka Ümit P, Cinel G. The effects of possible risk factors on morbidity in pediatric patients hospitalised with a diagnosis of pneumonia. Turk J Pediatr Dis. 2021;15:262‐333. 10.12956/tchd.688129 [DOI] [Google Scholar]
- 25. Kirolos A, Blacow RM, Parajuli A, et al. Impact of childhood undernutrition on pneumonia mortality: a systematic review and network meta‐analysis. BMJ Glob Health. 2021;6(11):e007411. 10.1136/bmjgh-2021-007411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheraghi M, Salvi S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur J Pediatr. 2009;168(8):897‐905. 10.1007/s00431-009-0967-3 [DOI] [PubMed] [Google Scholar]
- 27. Wonodi CB, Deloria‐Knoll M, Feikin DR, et al. Assessment of risk factors for severe pneumonia in children: pneumonia etiology investigation for the child health study. Clinical Infectious Diseases. 2012;54(suppl 2):S124‐S131. 10.1093/cid/cir1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turel O, Tahmiscioglu S, Siraneci R, Gonen I, Aydogmus C, Hatipoglu H. Investigation of etiological factors and prognosis in children with recurrent pneumonia. J Gynaecol Obstet Pediatr Pediatr Surg. 2012;4(1):24‐30. 10.5222/JOPP.2012.024 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


