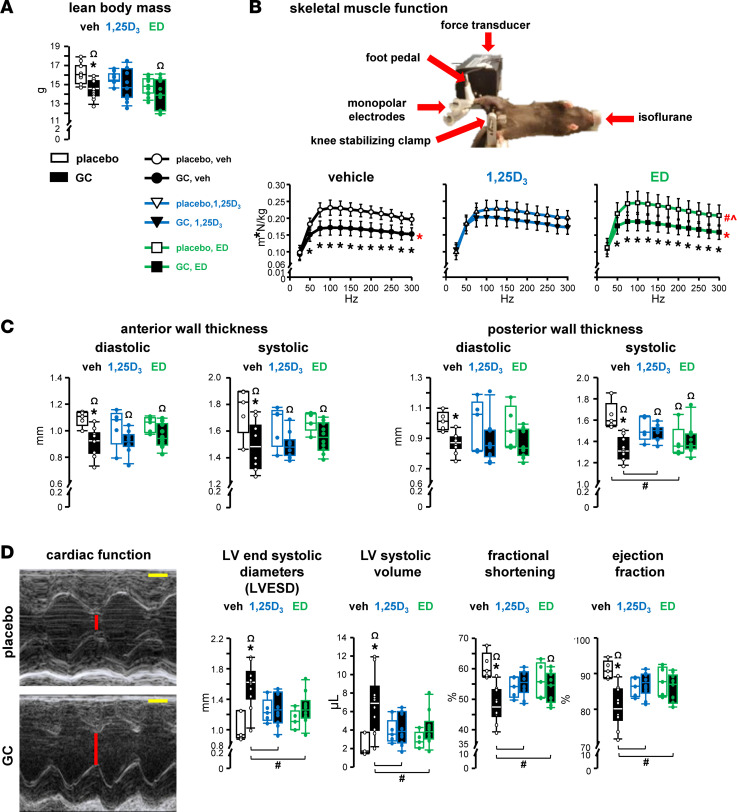
Figure 4. VDR ligands partially protect against skeletal and cardiac muscle dysfunction induced by GC.
Mice were implanted with 2.1 mg/kg/d prednisolone or placebo slow-release pellets and gavaged 5 times per week with 50 ng/kg/d 1,25D3, ED, or vehicle for 8 weeks. (A) Lean body mass and (B) skeletal muscle function, as assessed by plantarflexion torque in vivo testing measured after 4 weeks of the indicated treatments. n = 10–12. *P < 0.05 vs. corresponding placebo treated, by 2-way ANOVA for A and by 2-way repeated-measures ANOVA, Tukey’s post hoc test for B. Main group effects are indicated by red symbols: red *P < 0.05 all corresponding placebos vs. all corresponding GC, red #P < 0.05 all corresponding vehicles vs. all corresponding EDs, red ^P < 0.05 all corresponding 1,25D3s vs. all corresponding EDs by 2-way repeated-measures ANOVA, Tukey’s post hoc test. (C) Left ventricle (LV) wall thickness of the anterior and posterior surfaces at diastole and systole, as measured by Vevo2100 Imaging System ultrasound biomicroscopy system in vivo. (D) Representative images, LV end systolic diameters, LV systolic volume, fractional shortening, and ejection fraction generated from ultrasound echocardiograms (scale bars: 1 mm). n = 5 placebo-treated, n = 10–12 GC-treated. *P < 0.05 vs. corresponding placebo treated, #P < 0.05 vs. corresponding vehicle treated, ^P < 0.05 vs. corresponding 1,25D3 treated by 2-way ANOVA, Tukey’s post hoc test, ΩP < 0.05 vs. placebo and vehicle-treated controls by 1-way ANOVA, Dunnett’s method post hoc test.