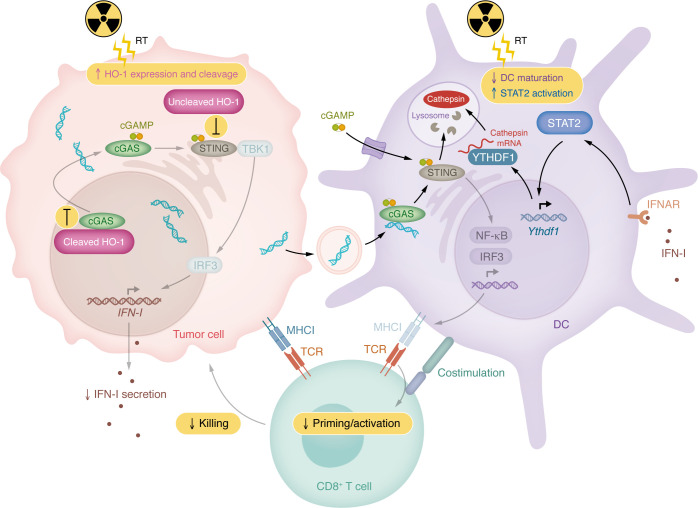
Figure 1. STING suppression after RT limits antitumor T cell immunity.
Zhang et al. (11) demonstrated that RT induces HO-1 expression and cleavage in cancer cells. This process leads to two mechanisms by which HO-1 suppresses cGAS/STING signaling. First, cleaved HO-1 localizes to the nucleus and binds to cGAS to prevent its nuclear export — preventing cGAS-dependent production of cytosolic cyclic dinucleotides (CDNs), such as cGAMP, that activate STING. Second, uncleaved HO-1, which retains its transmembrane domain, remains at the ER and directly interacts with STING to prevent its oligomerization, ER lumen curvature, and interaction with TBK1, impeding downstream signaling from STING. Together, these effects reduce the amount of intracellular CDN production and IFN-I secretion, limiting the delivery of these key immunostimulatory molecules to DCs and other cells within the tumor microenvironment. Wen et al. (10) discovered that RT induces the expression of YTHDF1 through IFN-I:IFNAR–dependent STAT2 activation, which directly binds to the Ythdf1 promoter to promote YTHDF1 transcription in DCs. YTHDF1 then binds to cathepsin mRNA to support its translation, leading to an overall increase in cathepsin expression and presence in lysosomes. STING activation, elicited by either engulfed cancer cell DNA recognized by cGAS or through import of extracellular CDNs, leads to the oligomerization of STING and production of vesicles from which productive STING signaling occurs. These vesicles are degraded by cathepsins in lysosomes, attenuating STING signaling. Ultimately, cancer cell and DC-intrinsic STING signaling can induce DC maturation/activation that leads to priming of tumor antigen–specific CD8+ T cells by DCs that recognize and kill cancer cells. Both HO-1 and YTHDF1 impair DC maturation, cross-presentation, and/or antitumor T cell immunity after radiation.