Abstract
With rapid expansion of cannabis legalization worldwide, rates of cannabis use and cannabis use disorder (CUD) are increasing; the need for safe and effective medications to treat CUD is urgent. This narrative review evaluates evidence for promising pharmacotherapies to treat CUD from randomized, placebo-controlled trials. Pharmacotherapies for CUD are categorized based on compound targets (e.g., cannabinoid receptor 1 [CB1] agonists such as nabilone, serotonergic compounds such as bupropion, GABAergic compounds such as zolpidem) and outcomes are organized by predetermined withdrawal symptoms, cannabis craving, and cannabis relapse/use. Most promising pharmacotherapies for CUD are drugs that act on the endocannabinoid system and specifically at the CB1 receptor. Priority populations such as females, certain racial/ethnic groups, and age groups experience a different course of CUD progression, symptoms, and drug effects that are important to consider when evaluating outcomes related to CUD. Possible explanations for these disparities are explored, along with the clinical trials that explore these demographics in treating CUD with pharmacotherapies.
Key Points
| The most effective treatments for decreasing cannabis withdrawal, craving, and relapse are agents that act at the cannabinoid 1 (CB1) receptor. |
| Limited but promising evidence has emerged on fatty acid amide hydrolase (FAAH) inhibitors for decreasing withdrawal and relapse. |
| Not all populations experience cannabis use disorder equally, and population diversity impacted by sex, race/ethnicity, and age should be considered when evaluating treatments. |
Introduction
In 2020, 49% of US adults aged ≥ 18 reported cannabis use in the past year and 12% reported use in the past month [1]. A subset of people who use cannabis develop problematic use patterns and what is known as cannabis use disorder (CUD), classically described as “continued use of cannabis despite impairment in psychological, physical, or social functioning” [2]. In 2021, 5.8% or 16.3 million people 12 and older reported a past-year CUD in the USA, and 16.1% of these individuals were classified as having a severe CUD (6 or more DSM-5 criteria met) [3]. Given the prevalence of cannabis use, it is important to understand its potential beneficial and negative health effects. Cannabis can be used for therapeutic effects such as pain relief in patients with chronic pain, use as an antiemetic in patients undergoing chemotherapy, as well as reduced spasticity in patients with multiple sclerosis [4–8]. However, for those who use cannabis for non-medical reasons and struggle with CUD, there are currently no FDA-approved pharmacotherapies. Universal behavioral treatments for managing substance use disorders can be applied to CUD such as Motivational Enhancement Therapy and Cognitive Behavioral Therapy [9], Contingency Management [10], and some are able to reduce their use without an intervention [11]. While these strategies range in success rates (15–45% at follow-up) [12–15], certain populations may benefit more or less from these strategies and pharmacotherapies may prove to be more effective alone or in combination with other behavioral therapies.
Cannabis contains over 140 phytocannabinoids; delta-9-tetrahydrocannabinol (THC) is the primary psychoactive and intoxicating component of the cannabis plant, is responsible for abuse liability and risk for CUD [2]. Cannabis use disorder is a diagnosis from the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) defined by the presence of ≥ 2 symptoms that correspond to a classification of mild (2–3 symptoms), moderate (4–5 symptoms), or severe (6+ symptoms) [16]. Symptoms include impaired control (e.g., strong craving and consuming more cannabis than intended), social problems (e.g., more time and effort spent obtaining cannabis that subtracts from other activities), risky use despite known consequences, and physiological and pharmacological drug effects (e.g., withdrawal symptoms when abstaining and tolerance to drug effects leading to increased use over time). Patients may present with different symptoms that qualify a diagnosis for CUD and severity of CUD is likely affected by strength of cannabis use (as defined by THC concentration), frequency of use, and route of administration [17, 18]. For those who seek treatment, successful resolution of CUD symptoms may vary as a function of CUD severity, concurrent use of other substances, psychiatric comorbidities, and patient demographics (refer to Sect. 3. Considering priority populations in the exploration of pharmacotherapies for CUD).
A subset of people who use cannabis frequently experience cannabis withdrawal syndrome (CWS), a syndrome characterizing the psychological and behavioral symptoms associated with cannabis withdrawal after cessation of use. Withdrawal symptoms typically develop within 24 h after cessation of cannabis use and peak two to three days after cessation; some symptoms can be prolonged in nature and not resolve even weeks after abstinence. Hallmark symptoms characterizing CWS are irritability, anxiety, sleep difficulty, decreased appetite, depressed mood, and physical discomfort (i.e., abdominal pain, tremors, sweating, fever, chills, or headache) [19–22]. In a nationally representative sample of people who use frequently with CWS from the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III), the most common symptoms reported were anxiety (76%), hostility (72%), sleep difficulty (68%), and depressed mood (59%) [23]. Withdrawal symptoms vary with respect to the population studied, frequency of cannabis use, and treatment-seeking status. For example, in a large population-based survey among non-treatment seekers who use cannabis at least three times a week, 12% of adults who used cannabis endorsed past year CWS [23], but a meta-analysis reported CWS to be much higher in outpatient samples (54%) and even higher in inpatient samples (87%) seeking treatment for CUD [24]. Estimates for CWS were higher for populations of treatment-seeking individuals compared to nontreatment-seeking, possibly reflecting greater symptomology among those with greater severity of CUD [24]. Reducing withdrawal symptoms is hypothesized to potentially aid in successfully establishing abstinence or reduced cannabis use [25, 26]. As such, pharmacotherapies that can reduce withdrawal symptoms have been probed as potential pharmacotherapies for CUD, while others focus on different aspects inherent in CUD symptomatology, which is continued use, or relapse once abstinence is achieved, regardless of the effects of such pharmacotherapies on CWS.
Neurobiology
As cannabis has complex pharmacology with many different chemical constituents, CUD is associated with an array of altered neurotransmitter systems. The two most studied phytocannabinoids, THC, a partial cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2) agonist, and cannabidiol (CBD), have diverse mechanisms of action attributed to their effects [27]. Liability for cannabis misuse and development of CUD is attributed to THC [28, 29]. By engaging the endogenous cannabinoid system, THC may downregulate CB1 receptors in the brain, as there is evidence from preclinical studies and clinical studies pointing to reduced CB1 receptors after chronic THC exposure [30, 31]. The CB1 receptor is downregulated in brain regions responsible for a host of experiences and abilities such as cognitive processing and decision making, motivation, risk/reward behaviors, sensory perception, and memory (i.e., the anterior cingulate cortex, hippocampus, insula, occipital cortex, parietal cortex, posterior cingulate cortex, prefrontal cortex, parahippocampal gyrus, and the lateral temporal cortex) [31]. Reductions in cannabinoid receptors may disrupt endocannabinoid homeostasis; pharmacologically restoring this homeostasis is one of the treatment strategies that will be reviewed in this paper. Delta-9-tetrahydrocannabinol also engages indirect targets other than the CB1 receptor that may be useful targets for treating CUD. Other neurotransmitter systems secondary to the effects at the CB1 receptor may also be dysregulated in CUD and provide additional targets for pharmacotherapies, such as the serotonin, endogenous opioid, nicotinic, Gamma-aminobutyric acid (GABA), and other systems [32]. These systems will also serve to define the broader organizational structure of this review.
Cannabis Regulation and Factors Related to CUD
With changing legislation increasing access to cannabis, rates of CUD may also increase. For example, legalization of non-medical cannabis use is associated with increases in cannabis use and rates of CUD [33]. As such, identifying safe and effective pharmacotherapies for CUD is increasingly important.
Risk factors influencing CUD directly and indirectly include specific genotype, as CUD is a polygenetic disorder with heritable risk factors [34], sex differences that influence predisposition to CUD and symptomology [35], as well as socioeconomic adversities [36]. For example, newly abstinent patients with CUD report that the two largest factors were using cannabis as a coping strategy and environmental/social influences [37]. Various factors contribute to CUD and understanding where shared pathologies and vulnerabilities intersect may be useful in considering pharmacological approaches and are discussed in this review.
Objective
There is urgent need for a safe and effective pharmacotherapy to treat CUD. The THC concentrations in cannabis are at a historical high [38] and more states within the USA are legalizing adult use of cannabis, increasing the availability of cannabis and risk for CUD. The goal of this paper is to highlight the evidence of previously evaluated and newer promising therapies for CUD as well as knowledge gaps that need to be addressed in order to understand the attributes that may be important in considering whether a pharmacotherapy may have potential for treating CUD. As such, this review provides an updated comprehensive description of clinical, placebo-controlled studies investigating candidate pharmacotherapies according to their general mechanisms of action that have undergone rigorous testing to determine their safety and potential effectiveness to treat CUDs. Neurotransmitter systems that have been targeted in the development of pharmacotherapies for CUD include the cannabinoid, serotonin, opioid, nicotinic, GABA, and α2-adrenergic receptor systems. A brief explanation of the preclinical justification for the potential of each of these targets that informed human studies of the particular drug class followed is provided. Next, evidence that supports or refutes the potential for these pharmacotherapies for treating CUD is described. We also highlight the knowledge gaps that exist and need to be investigated before clinical development of a potential pharmacotherapy can proceed. Novel pharmacotherapies for CUD that are early in development will also be discussed. Finally, we discuss the need for future clinical studies to specifically address safety and efficacy of potential CUD pharmacotherapies in vulnerable populations.
Clinical Studies of Pharmacotherapies for CUD
Studies determining the potential safety and effectiveness of pharmacotherapies for CUD have been performed in both volunteers with CUD who are not seeking treatment [39–54] and in patients who are seeking treatment [55–77]. Initial studies of specific pharmacotherapies are usually first carried out in nontreatment-seekers and are tested under non-abstinent and cannabis abstinent conditions. During non-abstinent conditions, studies are designed to understand how the agent may impact the acute effects of cannabis, including intoxication and positive-subjective drug effects such as drug liking and good drug effect. These studies help to determine whether the pharmacotherapy will impact cannabis direct effects relevant to CUD (i.e., intoxication, abuse liability). During abstinence, many studies are designed to assess how these agents impact symptoms of cannabis withdrawal, cannabis craving, and cannabis self-administration using a laboratory model of relapse where the ‘cost’ of initiating self-administration is high [46, 49, 51, 53]. These studies inform the extent to which the drug pharmacotherapy may be effective in people who are seeking treatment for CUD. The primary outcome measured in studies with treatment-seekers is usually reductions in cannabis use or abstinence. In both study designs, adverse effects of the agents are also explored; these may include sleep measures, cognitive performance, and mood. Here, we describe test medications that have been assessed primarily under placebo-controlled conditions in non-treatment and treatment-seekers according to pharmacological target.
Agents that Act on the Cannabinoid System and Other Cannabinoids
Cannabinoid 1 Receptor Agonists
Cannabinoid 1 (CB1) receptor agonists such as synthetic THC (i.e., dronabinol), nabilone, and nabiximols (plant-derived THC and cannabidiol [CBD]) have been investigated for the treatment of CUD, to potentially decrease cannabis withdrawal symptoms during abstinence and reduce cannabis use (Table 1). Across species, the CB1 receptor mediates the rewarding and reinforcing effects of THC [78–80]. The CB1 receptor also mediates cannabinoid dependence that occurs after repeated administration of THC in animals [81, 82]. This is evidenced by the ability of CB1 receptor antagonists to precipitate withdrawal in laboratory animals treated repeatedly with THC [83, 84]; THC and other CB1 receptor agonists dose-dependently reduce behavioral and physiological markers of CB1 receptor antagonist-induced precipitated withdrawal [85]. Extending the finding in preclinical laboratory animal studies showing that CB1 receptor agonists reduce THC withdrawal during abstinence, human studies examined the extent to which CB1 receptor agonists would reduce cannabis withdrawal symptoms during abstinence. In nontreatment-seeking volunteers who used cannabis frequently, dronabinol (10 mg 5 times daily in one study; 10 or 30 mg three times a day [TID] in another) reduced hallmark symptoms of cannabis withdrawal such as sleep disruption, reduced cannabis craving, and reduced cannabis withdrawal score where measured (Table 2) [39, 40]. Similarly, among treatment-seeking individuals, dronabinol (20 mg twice daily [BID]) also improved withdrawal symptoms [55]. Contrary to these findings, one study administering 20 mg dronabinol TID to nontreatment-seeking volunteers did not improve cannabis craving or relapse and worsened some measures of sleep quality (sleep latency) [41]. Multiple studies [39, 40, 55] support that dronabinol may reduce cannabis withdrawal symptoms and encourage further CB1 agonist studies. Neither treatment-seekers nor nontreatment-seekers reported improvements in cannabis relapse, but both reported improved withdrawal symptoms, highlighting the ways in which CB1 agonists may help to blunt the cannabis withdrawal symptoms experienced during early abstinence.
Table 1.
Methods and drug characteristics of the studies included in this review
| Drug | Participant characteristics | Dose conditions | ||
|---|---|---|---|---|
| N completers; sex% male | Treatment-seeking status; age; type of study | Cannabis use eligibility; average cannabis usea | Dose; route of administration; duration of treatment | |
| Cannabinoid 1 receptor agonists | ||||
| Dronabinol [39] | 7; 100% | Nontreatment-seeking; 24; within-subject | NA; 6.2 d/w | 50 mg (10 mg 5 times/day); oral; 6 d |
| Dronabinol [40] | 8; 75% | Nontreatment-seeking; 32.5; within-subject | ≥ 25 d/month in past 6 months and ≥ 2-year history of regular use; 28.5 d/month | 30 mg (10 mg/TID) and 90 mg (30 mg/TID); oral; 5 d |
| Dronabinol [55] | 156; 79–85% | Treatment-seeking; 36.9–38.4; parallel-group | ≥ 5 d/w in past 28 d; 30/30 d | 40 mg (20 mg BID); oral; 11 w |
| Nabilone [86] | 12; 75% | Unknown; 25.1; between-subject | Cannabis dependence; 28.1/30 d | 2 mg; oral; 10 w |
| Nabilone [42] | 11; 73% | Nontreatment-seeking; 30; within-subject | 5 d/w; 6.9 d/w | 6 mg and 8 mg (4 mg BID); oral; 7 d |
| Nabiximols [56] | 51; 76% | Treatment-seeking; 35.4; between-subject | Cannabis dependence; 20 years of cannabis use | Max 32 actuations per day; oromucosal spray; 6 d |
| Nabiximols [57] | 128; 77% | |Treatment-seeking; 35; parallel-group | Cannabis dependence; 25.7/28 d | Max 32 actuations per day; oromucosal spray; 12 w |
| Cannabinoid 1 receptor antagonists | ||||
| Rimonabant [89] | 63; 100% | Nontreatment-seeking; 23.8–29.3 across groups; between-subject | ≥ 1 year history of cannabis use; varies per treatment group | 1, 3, 10, 30, 90 mg; oral; single dose |
| Rimonabant [90] | 36; 100% | Nontreatment-seeking; 28.9; parallel-group | History of smoked cannabis; 20.3/30 d | 40 mg or 90 mg; oral; 15 d 40 mg, or 14 d 40 mg and one day 90 mg |
| Fatty acid amide hydrolase inhibitors | ||||
| PF-04457845 [99] | 70; 100% | Desire to quit ranged; PbO 27.5, PF-04457845 28.5; parallel-group | ≥ 2 years of regular use; 88–89% reported > 1000 d used/lifetime | 4 mg; oral; 3 w |
| Cannabidiol | ||||
| Cannabidiol [43] | 31; 55% | Nontreatment-seeking; 29.1; within-subject | ≥ 4 times used/w for four w; 6.5/7 d | 200, 400, 800 mg; oral; Single dose |
| Cannabidiol [58] | 48 (stage 1) and 34 (stage 2); 70–75% | Motivated to quit; 24.9–27.4; parallel-group | ≥ Moderate severity of CUD; NA | 200 (100 mg BID), 400 (200 mg BID), 800 mg (400 mg BID); oral; 4 w |
| Ligands that act via the serotonin system | ||||
| Lorcaserin [47] | 15; 73% | Nontreatment-seeking; 30.9; within-subject | ≥ 6 d/w for four w; 6.9 d/w | 20 mg (10 mg BID); oral; 13 d |
| Buspirone [60] | 50 (intention to treat sample); 83–96% | Treatment-seeking; 29.5–33.4; between-subject | Cannabis dependence; use on 90% of past 90 d | 60 mg; oral; 12 w |
| Buspirone [66] | 175; 77% | Treatment-seeking; 24; between-subject | Cannabis dependence; 85.2/90 d | 60 mg; oral; 12 w |
| Nefazodone [44] | 7; 86% | Nontreatment-seeking; 30; within-subject | Regular use; 6.4 d/w | 450 mg; oral; 26 d |
| Venlafaxine extended release [64] | 103; 69–79% | Treatment-seeking; 34.2–35.9; between-subject | Cannabis dependence; 27.4 d/month | Up to 375 mg; oral; 11 w |
| Bupropion-SR [59] | 10; 80% | Treatment-seeking; 27; Crossover design | Regular use; 6 d/w | 300 mg; oral; 28 d |
| Bupropion-SR [62] | 9; 56% | Treatment-seeking; 31.2; between-subject | 5 d/w; daily | 150 mg BID; oral; 21 d |
| Bupropion-SR and nefazodone [119] | 106; 63–93% | Treatment-seeking; 31–34; between-subject | Cannabis dependence; 27–29 d/past 30 | Nefazodone or bupropion SR; nefazodone 300 mg BID, bupropion SR 150 mg BID; oral; 10 w |
| Mirtazapine [45] | 11; 100% | Nontreatment-seeking; 27; within-subject | NA; 6.9 d/w | 30 mg; oral; 14 d |
| N-Acetylcysteine [63] | 116; 84% | Treatment-seeking; 18.9; parallel-group | Regular use and cannabis dependence; 22.6 d/past 30 | 600 mg BID; oral; 8 w |
| N-Acetylcysteine [68] | 302; 72% | Treatment-seeking; 30.3; parallel-group | Positive urine screen and cannabis dependence; 26 d/past 30 | 600 mg BID; oral; 12 w; |
| Quetiapine [46] | 14; 88% | Nontreatment-seeking; 26; within-subject | Regular use; 6.6 d/w | 200 mg; oral; 15 d |
| Quetiapine [69] | 130; 79% | Treatment-seeking; 32.9; between-subject | 5 d/w; 26.6 d/past 28 | 300 mg; oral; 12 w |
| Escitalopram [65] | 52; 75% | Treatment-seeking; 32.7; between-subject | Cannabis dependence; 42/52 THC positive | 10 mg; oral; 9 w |
| Vilazodone [67] | 76; 79% | Treatment-seeking; 22.2; between-subject | Cannabis dependence; use on 81.9% of past 30 d | Up to 40 mg; oral; 8 w |
| Ligands that act via the opioid system | ||||
| Naltrexone [124] | 29; 52% | Nontreatment-seeking; 28.1; within-subject | Use ≥ 4 times/w; 6.7 d/w | 0, 12, 25, 50, or 100 mg; oral; single administration |
| Naltrexone [48] | 51; 86% | Nontreatment-seeking; 28.6–31.6; between-subject | ≥ 4 times used/w for four w; 6.3–6.4 d/w | 50 mg; oral; 16 d |
| Ligands that act via the nicotinic system | ||||
| Varenicline (and nabilone) [49] | 46 completed inpatient; 78–86% | Nontreatment-seeking; 31.4–32.7; between-subject | Use ≥ 6 d/w for past 4 w; 6.9–7 d/w | Varenicline and nabilone; varenicline 1 mg BID, nabilone 4 mg BID; oral; varenicline titrated to 1 mg BID for 8 d, then 6 d nabilone |
| Varenicline [77] | 72; 68% | Treatment-seeking; 30.2; between-subject | Use ≥ 3 d/w; 92.9% d of use/past 90 d | Up to 1 mg BID; oral; duration of treatment; 6 w |
| Ligands that act via the GABA system | ||||
| Divalproex [39] | 7; 86% | Nontreatment-seeking; 26; within-subject | NA; 6.6 d/w | 1500 mg/day; oral; 29 d |
| Divalproex sodium [70] | 13; 92% | Treatment-seeking; 31.5; crossover design | Cannabis dependence; use on 6.8–6.9 d/w | Up to 2000 mg; oral; 2 w |
| Zolpidem extended release [50] | 20; 85% | Nontreatment-seeking; 29; within-subject | ≥ 25 d/month; 4×d | 12.5 mg; oral; 3 d |
| Zolpidem extended release [73] | 65; 65% | Treatment-seeking; 32; between-subject | > 50 d/past; 28–29 d/past 30 | 12.5 mg; oral; 12 w |
| Zolpidem [51] | 11; 100% | Nontreatment-seeking; 27.5; within-subject | ≥ 5 d/w for past 4 w; 6.8 d/w | Nabilone and zolpidem; zolpidem 12.5 mg, or zolpidem 12.5 mg and nabilone 3 mg BID; oral; 8 d |
| Gabapentin [71] | 50; 88% | Treatment-seeking; 33.9; parallel-group | Cannabis dependence; 11.6 years daily cannabis smoking | 1200 mg (two 300 mg and one 400 mg dose); oral; 12 w |
| Lithium carbonate [61] | 38; 66% | Treatment-seeking; 40.5; between-subject | Cannabis dependence; daily use | 500 mg BID; oral; 8 d |
| Topiramate [72] | 66; 46–50% | Treatment-seeking; 18.8–20.3; between-subject | ≥ 2 times/w in past 30 d; use on 70.22–70.94% d | 200 mg; oral; 6 w (2 w of 200 mg) |
| Baclofen [45] | 10; 100% | Nontreatment-seeking; 29; within-subject | NA; use on 7.6 d/w | 20 mg TID, 30 mg TID; oral; 16 d |
| Ligands acting on α2-adrenergic receptors | ||||
| Lofexidine (and oral THC) [41] | 8; 100% | Nontreatment-seeking; 29; within-subject | NA; 7 d/w | THC and lofexidine (separate and combined); THC 20 mg TID, lofexidine 2.4 mg (dose disbursement varied); oral; 7 d |
| Lofexidine (and dronabinol) [74] | 122; 64–74% | Treatment-seeking; 34.8–35.4; between-subject | ≥ 5 d/w; 7 d/w | Dronabinol and lofexidine; dronabinol 20 mg TID, lofexidine 0.6 mg TID; oral; 10 w |
| Guanfacine [52] | 15; 87% | Nontreatment-seeking; 30; within-subject | ≥ 6 d/w; 6.9 d/w | 6 d 1 mg, 4 d 1.5 mg, 9 d 2 mg (inpatient); oral; inpatient 9 d |
| Drugs on the horizon and other targets | ||||
| Celecoxib [53] | 15; 80% | Nontreatment-seeking; 31.3; crossover design | ≥ 6 d/w; 6.9 d per w | 200 mg BID; oral; 11 d |
| Oxytocin [146] | 16; 75% | Nontreatment-seeking; 23.3; between-subject | Cannabis dependence; use on 87.6–90.8% | 40 IUs; intranasally |
| Oxytocin [76] | 16; 63% | Treatment-seeking; 25.5; between-subject | Cannabis dependence; NA | 40 IUs; intranasally; 3 sessions over 4 w |
| Atomoxetine [75] | 19; 76% | Treatment-seeking; 29.9; between-subject | Cannabis dependence; use on 85.9% d in past 90 | Up to 100 mg; oral; 12 w |
| AEF0117 [54] | 26; 97% | Nontreatment-seeking; 32; two independent cohorts, mixed model | With CUD; 6.9 d/w | 0.06 or 1 mg; oral; 5 d |
This table reports the primary methods of study experiments, participant demographics, and drug treatment details in published studies
BID twice a day, CUD cannabis use disorder, d day(s), d/w days per week, NA not applicable, PbO placebo, THC delta-9-tetrahydrocannabinol, TID three times a day, IU international units
aTo minimize variability in reporting units, cannabis use eligibility and cannabis use at baseline were reported in units of time where possible and not in amounts of products consumed
Table 2.
Outcome measures assessed with withdrawal and other factors categorized by drug and mechanism of action
| Drug (citation) TS or NTS | Cannabis withdrawal / associated symptoms | Cannabis craving | Cannabis relapse/use | ||||
|---|---|---|---|---|---|---|---|
| Composite cannabis withdrawal | Anxiety | Depressed mood | Sleep disruption | Reduced food intake | |||
| Cannabinoid 1 receptor agonists | |||||||
| Dronabinol [39] NTS | ↓a | ↓b | ↓ | ↓ | ↓ | ||
| Dronabinol [40] NTS | ↓ | ↓ | ↓ | ||||
| Dronabinol [41] NTS | ↑ | ↑ | – | – | |||
| Dronabinol [55] TS | ↓ | – | |||||
| Nabilone [42] NTS | –a | ↓ | ↓ | ↓ | ↓ | ||
| Nabiximols [56] TS | ↓ | –c | ↓d | – | ↓ | – | |
| Nabilone [86] | –e | –f | – | – | |||
| Nabiximols [57] TS | – | – | ↓ | ||||
| Fatty acid amide hydrolase inhibitors | |||||||
| PF-04457845 [99] | ↓ | ↓a | ↓b | ↓ | ↓ | ||
| Cannabidiol | |||||||
| Cannabidiol [43] NTS | – | ||||||
| Cannabidiol [58] TS | ↓ | ↓e | –h | ↑ | ↓ | ||
| Serotonergic compounds | |||||||
| Bupropion-SR [59] TS | ↑b | ↑ | – | ||||
| Nefazodone [44] NTS | ↓a | –b | – | – | |||
| Buspirone [60] TS | – | –i | – | – | |||
| Bupropion-SR and nefazodone [119] TS | – | –i | – | ↓ | |||
| Mirtazapine [45] NTS | –a | ↓ | ↓ | – | – | ||
| Bupropion-SR [62] TS | – | –e | –h | – | ↓ | ||
| N-acetylcysteine [63] TS | ↓ | ||||||
| Quetiapine [46] NTS | ↓ | ↓ | ↑ | ↑ | |||
| Venlafaxine [64] TS | –j | ↑ | |||||
| Escitalopram [65] TS | – | –k | –h | – | |||
| Buspirone [66] TS | – | – | |||||
| Vilazodone [67] TS | ↓ | – | |||||
| N-acetylcysteine [68] TS | – | ||||||
| Quetiapine [69] TS | ↓ | – | – | ↓ | |||
| Lorcaserin [47] NTS | –a | –g | ↑ | – | ↓ | ↓ | |
| Opioidergic compounds | |||||||
| Naltrexone [48] NTS | ↑ | – | ↓ | ||||
| Nicotinic compounds | |||||||
| Varenicline [49] NTS | ↓a | ↓g | ↑ | – | – | ||
| Varenicline and nabilone [49] NTS | –a | ↓g | ↓ | – | – | ||
| Varenicline [77] TS | – | – | ↓ | ||||
| GABAergic compounds | |||||||
| Divalproex [39] NTS | ↑a | ↓ | ↓ | ↓ | |||
| Divalproex [70] TS | – | – | |||||
| Baclofen [45] NTS | – | – | – | – | |||
| Zolpidem extended release [50] NTS | – | ↓ | – | ||||
| Gabapentin [71] TS | ↓ | ↓h | ↓ | ↓ | ↓ | ||
| Lithium [61] TS | – | –c | –d | – | – | ||
| Zolpidem [51] NTS | –a | ↓ | – | – | |||
| Zolpidem and nabilone [51] NTS | ↓a | ↓ | ↓ | ↓ | |||
| Topiramate [72] TS | –h | – | |||||
| Zolpidem extended release [73] TS | – | – | |||||
| α2-adrenergic compounds | |||||||
| Lofexidine [41] NTS | ↓ | ↓ | – | ↓ | |||
| Lofexidine and dronabinol [41] NTS | ↓ | – | ↓ | ↓ | |||
| Lofexidine and dronabinol [74] TS | – | – | |||||
| Guanfacine [52] NTS | ↓ | – | – | – | |||
| Drugs on the horizon and other targets | |||||||
| Atomoxetine [75] TS | – | – | |||||
| Oxytocin [146] | ↓l | ↓ | |||||
| Oxytocin [76] TS | ↓ | ||||||
| Celecoxib [53] NTS | –a | –g | ↓ | – | ↑ | – | |
| AEF0117 [54] NTS | –a | –g | – | – | ↓ | ||
This table reports the primary features of cannabis withdrawal measured across published studies investigating potential pharmacotherapies for cannabis use disorder (CUD). Some studies examined effects of the study medication during cannabis-abstinence periods and others utilized an active cannabis and placebo cannabis phase; data in this table are restricted to the cannabis-abstinence or placebo cannabis phase as this is when effects of study medication on withdrawal were assessed. Under each main heading, drugs are listed in chronological order by date of publication. Direction of arrows indicate results of effect for the majority of outcome measures from the withdrawal domain specified in the column header. Up arrows indicate an increase in the withdrawal symptom, down arrows indicate a decrease in the withdrawal symptoms (e.g., down arrow = decreased anxiety), and dashes represent that the symptom was measured but no change was observed. Subscales of composite cannabis withdrawal scales that capture anxiety, depression, and sleep disruption are not reported as separate outcomes unless these outcomes were assessed independent of the composite withdrawal score. Cells without indication mean there was not categorization for those studies
Anxiety was measured with the following scales: aAnxiety ratings from a subscale or single item from a visual analog scale (VAS), cAnxiety subscale from the Depression, Anxiety, and Stress Scale (DASS), eBeck Anxiety Inventory (BAI), iHamilton Anxiety Scale (HAM-A), kSpielberger State-Trait Anxiety Inventory (STAI), or the lanxiety rating on 1–10 Likert scale
Depressed mood was measured with the following scales: bMiserable ratings from a subscale or single item from a visual analog scale (VAS), ddepression subscale from the Depression, Anxiety, and Stress Scale (DASS), fQuick Inventory for Depressive Symptoms (QIDS), gMiserable subscale from visual analog scale (VAS), or the hBeck Depression Inventory (BDI), jHamilton Depression Rating Scale (HAMD)
NTS nontreatment-seekers, TS treatment-seekers
Nabilone, another CB1 receptor agonist, was investigated for reducing withdrawal symptoms and effects in a laboratory model of cannabis relapse in nontreatment-seekers (Table 1). A low dose of nabilone (2 mg) was not more effective than placebo at reducing cannabis use or craving [86]. However, higher nabilone doses (6 mg once daily and 4 mg BID) significantly attenuated cannabis withdrawal symptoms, including sleep disruptions, cannabis craving, and also reduced cannabis self-administration in a laboratory model of relapse in nontreatment-seeking volunteers compared to placebo [42]. However, it should be noted that the 4-mg BID dose had a negative impact on cognitive performance. These findings and one discussed later (see Sect. 2e: Agents that act via the GABA system) found nabilone plus zolpidem improved cannabis relapse [51], suggesting that nabilone may dose-dependently reduce cannabis craving, relapse, and abstinence-induced sleep problems. Clinical trials on nabilone in treatment-seeking individuals with CUD have yet to be conducted.
Two randomized clinical trials have examined the effects of nabiximols, an oromucosal spray containing 2.7 mg THC and 2.5 mg CBD per actuation (max. 32 sprays per day or 86.4 mg THC and 80 mg CBD in both studies), in treatment-seeking individuals (Table 1). The first study compared six days of nabiximols treatment to placebo during cannabis-abstinence on measures of cannabis withdrawal, craving, and later on cannabis use at follow-up [56]. During cannabis abstinence, nabiximols reduced withdrawal scores, decreased cannabis craving, and attenuated loss of appetite, but at the 28-day follow-up—after cessation of the treatment—there was no difference in cannabis use relative to placebo. The second study assessed the effects of nabiximols compared to placebo in combination with cognitive behavioral therapy counseling in a longer, 12-week study [57]. Compared to the placebo cohort, those who received nabiximols for 12 weeks used cannabis on 19 fewer days over the study period and continued to exhibit reduced cannabis use and abstinence after treatment cessation as measured at a 12- and 24-week follow-up [87]. To summarize, nabiximols reduced cannabis craving during periods of abstinence and reduced cannabis use when combined with a cognitive behavioral therapy. These studies support nabiximols as a potential pharmacotherapy that warrants further study to decrease the use of cannabis and associated withdrawal symptoms.
Replacement therapy with either synthetic or naturally derived CB1 receptor agonists are a promising strategy for treating withdrawal associated with CUD [40, 55, 56], as well as reducing craving [39, 40, 42, 56], and may potentially reduce cannabis use [42, 57]. However, it is unclear whether such treatment can be tapered and provide long-lasting abstinence without relapse, although similar strategies have proven successful for opioid use disorder with drugs such as buprenorphine and methadone [88]. Retention in treatment groups was similar to that of placebo, if not improved, and there were no serious adverse events related to the studies (Table 1).
CB1 Receptor Antagonists
Preclinical studies demonstrate that CB1 receptor antagonists/inverse agonists, such as rimonabant, reduced self-administration of THC in non-human primates [79, 80], and have therefore been explored for the treatment of CUD (Table 1). A study administering rimonabant (single dose of 1, 3, 10, 30, or 90 mg), a CB1 receptor antagonist/inverse agonist, found the highest dose (90 mg) blocked the intoxicating and positive subjective drug effects of cannabis compared to placebo in nontreatment-seeking volunteers [89]. Potential tolerance to rimonabant’s effects were later observed in a repeated dosing study where the drug was administered for 15 consecutive days [90]. Although intoxication and positive subjective effects from cannabis were significantly reduced on the 8th day of rimonabant treatment compared to placebo, these effects were reduced on the 15th day of treatment suggesting potential limited clinical utility for long-term treatment with CB1 receptor antagonists/inverse agonists.
The two mentioned studies reported that rimonabant was well tolerated and no associated serious adverse events were attributed to the drug, but rimonabant (approved in Europe as a weight-loss drug for obesity) was withdrawn worldwide in 2008 due to patient reports of increased depression, anxiety, and more severe psychiatric diagnoses including suicidal behavior [91]. In addition, six of the 42 originally enrolled subjects from the later-mentioned study [90] were excluded from analysis for not completing medication adherence, possibly demonstrating lack of clinical effectiveness. For these reasons, CB1 receptor orthosteric antagonists and inverse agonists have not been further explored. However, allosteric modulators of the CB1 receptor, are being investigated in preclinical studies, such as Org27569 and PSNCBAM-1 [92].
A novel ligand, AEF0117, acts as a signaling-specific inhibitor to inhibit intracellular effects in response to CB1 receptor binding. It is a synthetic pregnenolone analog, but with a more selective pharmacological profile of receptor binding [54]. Preclinical studies suggest that pregnenolone and CB1 receptors are involved in a negative feedback loop by which THC activates CB1 receptors, having a downstream effect of increased pregnenolone levels, that can then have an inhibitory effects on CB1 receptors [93]. Due to its more selective binding, AEF0117 may display similar behavioral outcomes as would a CB1 receptor negative allosteric modulator. Two doses of AEF0117 (0.6 and 1 mg) were investigated in nontreatment-seeking volunteers [54]. The highest dose, 1 mg, reduced cannabis self-administration in comparison to placebo. This type of action may be useful because it does not block all effects of CB1 receptor activation, as antagonists/inverse agonists do, but only minimizes activity of some intracellular processes.
Fatty Acid Amide Hydrolase Inhibitors
Fatty acid amide hydrolase (FAAH) is a hydrophobic enzyme that degrades signaling molecules, including endocannabinoids, at the cell membrane [94]. As such, FAAH inhibitors block the hydrolyses of endogenous cannabinoids including N-arachidonoylethanolamine (AEA) [95]. Fatty acid amide hydrolase inhibitors may be a therapeutic option to increase endocannabinoid levels in people with CUD, as frequent cannabis use is associated with lower cerebrospinal levels of AEA compared to infrequent use [96]. Additionally, because endocannabinoids are unstable and undergo rapid metabolism, manipulating their degradation through FAAH inhibitors provides an alternate mechanism to boost CB1 receptor agonism in a region-specific manner (i.e., only where AEA is already being produced, rather than global activation/inhibition with other agonists/antagonists). Preclinical studies have shown that FAAH inhibitors reduce behavioral and physiological markers of precipitated THC withdrawal (Table 1) [97]. The endocannabinoid anandamide may also play a protective role in psychotic symptoms [98].
To extend these preclinical findings, a published double-blind placebo-controlled study of a FAAH inhibitor, PF-04457845 (4 mg, ~ 4 weeks) was carried out in patients with CUD with a desire to stop using cannabis. Goals of this study were to determine if the candidate drug would reduce cannabis withdrawal during abstinence and if it would also reduce cannabis use. Participants randomized to the FAAH inhibitor exhibited reduced withdrawal symptoms such as depression, irritability, and anxiety and improved sleep compared to placebo; treatment with the FAAH inhibitor was also associated with reduced cannabis use compared to placebo [99]. This study provides important findings to advance further studies of the long-term potential of FAAH inhibitors to alleviate withdrawal symptoms and to reduce cannabis use. A phase 2 multi-site clinical trial is currently underway investigating PF-04457845 for CUD [100]. Although self-reported cannabis use after 8 weeks of treatment shows no improvement compared to placebo, differences in urine metabolites between treatment groups remain to be interpreted by statistical analysis. The reported FAAH inhibitor, PF-04457845, may hold promising potential as a pharmacotherapy with demonstrated reductions in cannabis withdrawal and cannabis use and no serious adverse events (Table 2).
Cannabidiol
Cannabidiol (CBD) is a non-intoxicating phytocannabinoid that has been investigated as a potential pharmacotherapy for CUD (Table 1). Cannabidiol has low binding affinities for CB1 and CB2 receptor but has been hypothesized to act on the endocannabinoid system as a FAAH inhibitor [101], a negative allosteric modulator of the CB1 receptor [102], and an inverse agonist of the CB2 receptor [103]. Other proposed mechanisms by which it may have therapeutic effects specifically within the context of CUD and other substance use disorders include its modulatory actions on glutamate-GABA systems [104] and its potential 5HT1A agonist effects [105]. Preclinical studies using alcohol and cocaine models of substance use disorder demonstrated that CBD decreased drug self-administration [106, 107]. In humans, one randomized placebo-controlled study found that CBD reduced cigarette smoking in humans, and another found CBD to reduce cue-induced craving in people with opioid use disorder [108, 109]. Such literature on CBD has primed the field to explore this phytocannabinoid as a potential therapeutic treatment for a broad range of drugs that are misused, including cannabis.
A few studies have rigorously tested CBD as a potential pharmacotherapy for CUD in treatment-seeking and nontreatment-seeking samples (Table 1). In people who used cannabis and were nontreatment-seeking, CBD (200, 400, and 800 mg) had no effect on the cannabis intoxication, positive subjective effects, or cannabis self-administration, compared to placebo [43]. In treatment-seeking volunteers, CBD (400 and 800 mg) had minimal effect in reducing cannabis use compared to placebo (less than 1 additional day of cannabis abstinence per week), although the 800-mg dose reduced CWS and anxiety [58]. Data suggest that CBD has the potential to improve CUD-associated symptoms and may assist in reducing cannabis use. CBD was well-tolerated without reported serious adverse events. Although it’s unclear what CBD’s pharmacological site of action may be targeting, Cannabidiol warrants further investigation for reducing cannabis use, withdrawal, and craving in samples motivated and not motivated to quit (Table 2).
Agents that Act Via the Serotonin System
Serotonin ligands, like some antidepressant and antipsychotic agents, have been investigated as potential pharmacotherapies for CUD (Table 1). In preclinical studies, the serotonin system contributes to neural mechanisms underlying the behavioral and cognitive deficits associated with chronic administration of THC and other CB1 receptor agonists. For example, chronic administration of a CB1 receptor agonist in adolescent rats elicits anxiety-like behavior that is associated with serotonergic hypoactivity [110]. Adolescent mice exposed to THC exhibit similar prefrontal cortex dysfunction and cognitive deficits to adults that can be prevented by serotonin receptors of type 6 (5HT6) receptor antagonists during concomitant treatment during adolescent exposure to THC [111]. These data suggest a modulatory effect of CB1 receptors on serotonin release and excitability of serotonin neurons [112]. The 5HT2C receptors are located in the same areas implicated in reward and motivation and overlap in expression on dopaminergic neurons [113]. Across species, lorcaserin (a serotonin 5HT2C agonist) reduces self-administration of a range of pharmacological reinforcers including nicotine, cocaine, and heroin [114–117]. The ability of 5HT2C agonists to reduce self-administration is likely due in part to its ability to inhibit dopamine release [118]. Modulating serotonin activity in humans with CUD has been investigated as a potential pharmacotherapy for improving withdrawal symptoms and relapse.
Lorcaserin was explored in a nontreatment-seeking CUD population and compared to placebo for its effects on cannabis withdrawal including cannabis craving, disruptions in sleep, and reduced caloric intake (Table 1) [47]. In nontreatment-seekers, lorcaserin (10 mg BID) decreased cannabis craving and cannabis self-administration compared to placebo. However, lorcaserin exacerbated sleep disruption associated with abstinence and impaired cognitive performance relative to placebo. In 2020, lorcaserin for weight loss was removed from the market due to potential cancer risks limiting its future utility. Because lorcaserin reduced cannabis craving and self-administration during abstinence, other 5HT2C agonists warrant further study as potential therapeutics for CUD.
Buspirone is an anxiolytic medication and partial 5HT1A agonist approved for general anxiety disorder that may alleviate symptoms of cannabis withdrawal and decrease likelihood of relapse. However, compared to placebo, buspirone (60 mg) did not reduce cannabis withdrawal symptoms, nor did it impact cannabis craving or cannabis use in participants seeking treatment for CUD [60, 66]. Although it was expected that buspirone would reduce anxiety associated with abstinence, the treatment groups did not differ on measures of anxiety, although a potential confound was that subjects had low baseline anxiety possibly as a function of exclusion criteria.
Studies have investigated serotonin and norepinephrine reuptake inhibitors (SNRI), such as nefazodone and venlafaxine, for cannabis abstinence and withdrawal (Table 1). In a nontreatment-seeking population, nefazodone (450 mg) reduced ratings of anxiety and muscle pain during withdrawal but had no effect on other withdrawal symptoms including irritability or sleep disruption. During abstinence, nefazodone also worsened cognitive performance compared to placebo on some endpoints [44]. Venlafaxine, another FDA-approved SNRI used to treat depression, social anxiety disorder, and cataplexy, was investigated for its potential to facilitate cannabis abstinence and improve depressive symptoms in a treatment-seeking and moderately depressed cohort. Extended-release venlafaxine (up to 375 mg) or placebo failed to alter mood or reduce abstinence rates compared to placebo [64]. Together these studies concluded that SNRIs are ineffective for treating CUD.
Bupropion, a norepinephrine–dopamine reuptake inhibitor (NDRI), is FDA-approved for depression, seasonal affective disorder, and cigarette smoking cessation. Nontreatment-seeking participants receiving bupropion-sustained release (SR; 300 mg/day) exhibited increases in subjective ratings of irritability, miserableness, restlessness, depression, lack of motivation, and decreased sleep quality during cannabis withdrawal compared to placebo [59]. In a treatment-seeking population, bupropion (150 mg BID) did not improve measures of withdrawal, depression, anxiety, sleep, or cognitive performance, but did reduce cannabis craving compared to placebo [62].
A comparison between the SNRI nefazodone (300 mg BID) and the NDRI, bupropion (150 mg BID) in treatment-seekers revealed that neither medication improved rates of cannabis abstinence or withdrawal symptoms relative to placebo [119]. As such, studies do not support the potential effectiveness of these classes of drugs for treating CUD (Table 2).
Mirtazapine has high affinity for 5HT2 and 5HT3 receptors and increases serotonin and noradrenergic neurotransmission by blocking presynaptic alpha-2 adrenoceptors, rather than acting on serotonin reuptake [120]. It is FDA-approved for major depressive disorder and earlier findings of this drug in patients with alcohol use disorder suggest that it may be helpful in reducing cannabis withdrawal symptoms [121]. In a nontreatment-seeking population mirtazapine (30 mg) improved cognitive performance, sleep, and caloric intake during cannabis abstinence, but failed to reduce cannabis craving and cannabis self-administration using a laboratory model of relapse (purchase of cannabis puffs; Table 2) [45].
Quetiapine is FDA-approved to treat schizophrenia, acute mania, and major depressive disorder. It is an antagonist at 5HT2A, dopamine D2, histamine H1, and adrenergic receptors. Because it has been shown to improve mood and sleep in patient populations, quetiapine has been investigated for CUD to possibly relieve similar symptoms during withdrawal (Table 1). In nontreatment-seeking volunteers, quetiapine (200 mg) decreased some physical symptoms of withdrawal compared to placebo. However, quetiapine increased cannabis self-administration using a laboratory model of relapse (purchase of cannabis puffs; Table 2) and worsened cognitive performance during withdrawal compared to placebo [46]. Among treatment-seekers, quetiapine (300 mg) was associated with modest reductions in cannabis use and a reduction with withdrawal symptoms compared to placebo [69]. Important differences between these studies that could contribute to divergent outcomes was the dose of the study medication (200 vs 300 mg) and treatment-seeking status.
Serotonin reuptake inhibitors that are FDA approved for depression have been tested for CUD, but like the SNRIs, do not appear effective in facilitating cannabis abstinence or reduced cannabis use. For example, escitalopram, a selective serotonin reuptake inhibitor (SSRI), was explored in a treatment-seeking population for abstinence-associated depression and anxiety (Table 1) [65]. Participants administered escitalopram (10 mg) revealed no treatment effect in any of the outcome measures compared to placebo. Finally, vilazodone (up to 40 mg) a SSRI and a 5TH1A receptor partial agonist, did not improve rates of abstinence or craving, apart from lowering scores on the purposefulness subscale of the Marijuana Craving Questionnaire (intention to use cannabis) compared to placebo in a treatment-seeking population [67]. In addition to the lack of promising outcomes on study measures (Table 2), those randomized to vilazodone attended fewer appointments compared to placebo further suggesting poor clinical potential.
To summarize, few serotonin agents are effective at reducing withdrawal-related measures of CUD or improving rates of abstinence of reductions in cannabis use. Buspirone, venlafaxine, mirtazapine, quetiapine, escitalopram, and vilazodone failed to improve measures of cannabis relapse/abstinence; N-acetylcysteine increased abstinence in adolescents, but this may be mediated via a non-serotonergic mechanism (Table 2). Buspirone, mirtazapine, quetiapine, and vilazodone did not decrease cannabis craving. Lorcaserin reduced craving and cannabis self-administration in a laboratory model of relapse in in-treatment-seekers, suggesting that agents acting as 5HT2C agonists may provide clinical potential for CUD that is unique to other serotonergic agents. Several of these test medications were associated with more adverse events compared to placebo such as dizziness, dry mouth, flushing/sweating, and cold-like symptoms for buspirone, loss of libido for venlafaxine [64], and upset stomach for bupropion [59]. Therapeutics that act on systems other than the serotonin system for treating CUD appear more promising.
Agents that Act Via the Opioid System
Compelling preclinical findings pointing to the involvement of the opioid system in CB1 receptor agonist modulated behaviors have spurred interest in probing μ-opioid antagonists as potential pharmacotherapies for CUD. For example, THC does not produce conditioned place preference in mice that lack the μ-opioid receptor suggesting that THC engages the endogenous opioid system [122]. In non-human primates, the μ-opioid receptor antagonist naltrexone, FDA-approved for alcohol and opioid use disorders, attenuates THC self-administration [123]. These preclinical findings suggest that opioid receptor antagonists may also reduce cannabis self-administration in humans and may be a potential treatment for CUD (Table 1). Unexpectedly, acute administration of naltrexone (12, 25, 50, or 100 mg) enhanced the positive, misuse-related subjective effects of cannabis in nontreatment-seeking people who use cannabis, suggesting that it increases cannabis abuse liability compared to placebo [124]. However, when naltrexone (50 mg) was administered for 16 days to nontreatment-seeking people who use cannabis it reduced the positive subjective ratings of cannabis and cannabis self-administration compared to placebo [48]. These findings point to naltrexone’s potential as a CUD treatment strategy when administered chronically rather than acutely; however, chronic administration was associated with mild adverse effects such as gastrointestinal upset, headache, and jitteriness relative to placebo. Despite the increase in adverse effects, there was not an increase in dropout rates among those receiving naltrexone compared to placebo. Further studies are needed to elucidate the time-dependent and dose-dependent relationship of opioid antagonists on the reinforcing properties of cannabis use, as well as its effects on cannabis withdrawal (Table 2).
Agents that Act Via the Nicotinic System
Many people who use cannabis have also used tobacco [125], and there is an established relationship between tobacco smoking and cannabis dependence, suggesting a shared pathology in receptor modification. Ninety percent of people who use cannabis also report current or past tobacco use [125]. A 4-year longitudinal study revealed that cigarette smoking mediates the relationship between cannabis use and cannabis dependance [126]. Inpatient studies support this finding, with cannabis relapse being increased for those who also smoke cigarettes compared to those who do not [127]. For this reason, studies have sought to assess the effects of FDA-approved medications for smoking cessation alone and in combination with other agents that may target cannabis withdrawal and relapse in people who have CUD and tobacco co-use.
For people who used cannabis and tobacco daily, varenicline, a partial nicotinic agonist that is FDA-approved for smoking cessation was assessed for its ability to reduce cannabis withdrawal and relapse (purchase of cannabis puffs) when administered alone and in combination with nabilone, a CB1 receptor agonist, described as having effects on cannabis withdrawal and relapse [42, 49]. A nontreatment-seeking sample was randomized to active varenicline (1 mg BID) or placebo for 15 days, then to active nabilone (4 mg BID or placebo) or placebo [49]. Varenicline alone reduced some withdrawal symptoms associated with tobacco withdrawal (ratings of “Miserable” and “Anxious”) compared to placebo, but did not affect most cannabis withdrawal symptoms, and in some instances worsened withdrawal (‘Anxious’). Nabilone in combination with varenicline reduced some cannabis withdrawal symptoms compared to placebo and varenicline alone. Cannabis relapse was unaffected by either medication. Dropout rates were comparable and there were no serious adverse events. A treatment-seeking sample, although not powered to detect differences in cannabis use, observed a greater change in abstinent days in participants randomized to varenicline (up to 1 mg BID for 6 weeks) compared to placebo [77]. It would be informative to have data on cognitive performance to add to the results of varenicline on improving mood during tobacco withdrawal, but this nicotinic agonist did not show promise for cannabis withdrawal and limited evidence for improving relapse (Table 2).
Agents that Act Via the GABA System
The reward circuitry includes the mesolimbic dopamine projection from the ventral tegmental area (VTA) to the nucleus accumbens. Drugs that are misused, including THC delivered to anesthetized rat models and in PET imaging in humans, modulate this circuit and increase extracellular dopamine, which has been associated with reward and reinforcement [128, 129]. In addition to dopamine neurons, the VTA contains both glutamatergic and GABAergic neurons, which tonically inhibit dopamine neurons, as such, inhibition of GABA neurons results in enhanced dopamine release. A proportion of VTA GABA neurons expresses CB1 receptors; activation of these receptors results in a decrease GABA release and subsequent increase in dopamine release [130]. Animal models demonstrate how THC administration can alter the expression of GABA in the prefrontal cortex, a region with reduced gyrification in adolescents who use cannabis compared to those who do not [131]. In rats, early THC exposure reduces GABA levels in the prefrontal cortex compared to control/vehicle [132]. Tiagabine, a GABA transporter 1 inhibitor, produces similar THC-like discriminative stimulus and subjective effects in humans, suggesting that in some cells THC-induced increases in GABA significantly contribute to the interoceptive and subjective experience of this CB1 receptor agonist [133]. Altering the GABA system is one way to indirectly control dopamine release and to potentially protect from the withdrawal associated with cannabis abstinence. For this reason, drugs that act on GABA have been investigated for therapeutic utility for CUD in addition to other substance use disorders (e.g., divalproex [39, 70], zolpidem [50, 51, 73], gabapentin [71], lithium [61], topiramate [72], and baclofen [45]).
Divalproex increases plasma GABA levels and is FDA-approved to treat seizures, acute mania, and to prevent migraine headaches. In a nontreatment-seeking sample, divalproex (1500 mg) decreased ratings of cannabis craving, but increased ratings of subjective effects of “anxious”, “irritable”, and “on edge” during cannabis abstinence compared to placebo [39]. Compared to placebo, participants were less social, gained weight, and performed worse on cognitive tasks when administered divalproex. In treatment seekers, divalproex (up to 2000 mg) showed no treatment effect on cannabis use or withdrawal compared to placebo [70]. Literature does not support divalproex as an effective medication for CUD.
Zolpidem has high affinity for the GABA-A alpha 1 subunit receptor, acting to enhance GABAergic inhibition and is FDA-approved to treat insomnia [134]. Sleep disruption is a hallmark symptom of cannabis withdrawal; therefore, zolpidem was assessed for its potential effects on cannabis withdrawal. While zolpidem (12.5 mg) failed to improve cannabis withdrawal scores, discomfort scores, or craving, it did improve some abstinence-induced sleep problems without affecting cognitive performance compared to placebo in nontreatment seekers (Table 2) [50].
In treatment-seeking participants administered the same dose (12.5 mg), no improvements in sleep disturbances or in cannabis abstinence rates compared to placebo were observed. In another study with nontreatment-seeking participants, zolpidem alone (12.5 mg) was compared to zolpidem (12.5 mg) with nabilone (3 mg) to examine outcomes of cannabis withdrawal and relapse [51]. Compared with placebo, zolpidem alone and combined with nabilone improved sleep, but cannabis relapse was reduced only when zolpidem was combined with nabilone. Subjective ratings of “Irritable” and “Anxious” were reduced by nabilone and zolpidem co-administration, but not zolpidem alone. Zolpidem appears effective for improving sleep disturbance but no other withdrawal-related symptoms of CUD; added benefits are evident when administered in combination with nabilone.
Gabapentin is a GABAergic modulating drug that has been explored for cannabis use and CUD symptoms (Table 1) [71]. Published findings of treatment-seeking participants administered gabapentin (1200 mg/day) experienced improved outcomes of depression, sleep, executive function, cannabis withdrawal scores, and decreased cannabis use compared to placebo. However, unpublished findings from a fully powered sample suggest gabapentin (11.22% negative cannabis urine drug screen) may be no more advantageous over placebo (8%) in achieving cannabis abstinence after 12 weeks of administration [135].
Other GABA modulators such as lithium, topiramate, and baclofen have been tested with less promising results as potential CUD treatments (Table 2). Lithium is FDA-approved for treating manic episodes of bipolar disorder, topiramate for epilepsy, and baclofen for muscle spasticity [136]. In a treatment-seeking population, lithium (500 mg BID), which increases GABAergic neurotransmission, did not improve withdrawal symptoms apart from individual scores on appetite loss, stomachache, and nightmares compared to placebo [61]. Topiramate increases GABA activity and is a GABA-A receptor agonist. In treatment-seeking adolescents, topiramate (200 mg) increased study drop out, worsened neurocognitive performance, and did not improve frequency of cannabis use; however, there was a reduction in grams of cannabis smoked compared to placebo [72]. Two doses of baclofen (20 mg TID, 30 mg TID), a GABA-B receptor agonist, were compared to placebo in a nontreatment-seeking population [45]. Baclofen did not improve relapse but decreased cannabis as well as cigarette craving in tobacco at the higher dose. Negative effects such as sleep disruptions, decreased cognitive performance, and increases in alcohol craving were also reported to provide little support for the use of baclofen for CUD. Apart from gabapentin, GABA modulators do not hold promise for treating cannabis withdrawal or reductions in cannabis use in adult or adolescent samples (Table 2).
Our review of ligands that act via the GABA system concludes that these medications are ineffective or the benefits do not outweigh the adverse events (Table 2). The most promising study showed gabapentin reduced cannabis use and craving without sacrificing adverse events and should be further investigated for cannabis intoxication and withdrawal symptoms such as anxiety and food intake. Divalproex may be useful for cannabis craving and topiramate may help reduce frequency of cannabis use, but both medications reported adverse events that may reduce efficacy and medication adherence. Although dopamine and GABA neurons are critical components of the reward system, modulating the GABA system does not appear effective for treating CUD without avoiding other problematic side effects.
Agents that Act on α2-Adrenergic Receptors
Alpha2-adrenergic receptor agonists have medical utility for hypertension, attention disorders, and are used to manage symptoms associated with opioid withdrawal [137, 138]. Preclinical models show that α2-adrenergic agonists increase excitability in the medial prefrontal cortex, and that CB1 receptor stimulation desensitizes these α2-adrenergic receptors [139]. The prefrontal cortex is one of many brain areas with downregulated CB1 receptors in people who use cannabis frequently [31]. Targeting desensitized receptors to increase neuron excitability with α2-adrenergic agonists may aid in relieving cannabis withdrawal symptoms.
Lofexidine is a α2-adrenergic receptor that is FDA-approved to manage opioid withdrawal symptom [136]. Because some opioid-withdrawal symptoms overlap and with symptoms of cannabis withdrawal, it has been investigated as potential pharmacotherapy for CUD. Over four treatment phases, participants not seeking treatment for their cannabis use were administered dronabinol (60 mg/day), lofexidine (2.4 mg/day), dronabinol with lofexidine, or placebo [41]. Relative to placebo, lofexidine alone and combined with dronabinol decreased cannabis relapse (double that of either medication alone), and improved sleep, where dronabinol alone did not reduce relapse. Dronabinol with lofexidine decreased symptoms such as restlessness, chills, upset stomach, and cannabis craving. Results support combining dronabinol with other pharmacotherapies to improve effectiveness both for cannabis withdrawal-associated symptoms and relapse. However, in an outpatient study enrolling treatment-seekers receiving dronabinol (60 mg/day) with lofexidine (2.4 mg/day), medication did not improve initiation to abstinence, withdrawal symptoms, or compared to placebo [74]. Differences between studies was the study population (treatment-seekers versus nontreatment-seekers), study design (outpatient vs inpatient) and outcome measures associated with cannabis use while undergoing the pharmacotherapy; the inpatient short study assessed cannabis use in a laboratory model of relapse after three days of forced abstinence with continuous measure of cannabis self-administration [41], whereas the outpatient study with treatment-seekers required that participants self-initiate abstinence prior to assessing relapse (continued abstinence) [74]. The drug combination may have successfully improved withdrawal and continued abstinence once abstinence initiation was achieved in a controlled setting (i.e., inpatient setting).
Similar to lofexidine, guanfacine is an α2-adrenergic agonist; it is FDA-approved for hypertension and attention deficit hyperactivity disorder (ADHD) and is suggested to have a superior safety profile to lofexidine [136]. In a nontreatment-seeking population, guanfacine (2 mg) did not reduce cannabis relapse, but did improve measures of withdrawal such as irritability, blood pressure, sleep, and cognitive performance compared to placebo [52]. This study offers support for targeting the α2-adrenergic receptors to reduce symptoms of cannabis withdrawal and outcomes should be investigated at a longer follow-up (Table 2).
The α2-adrenergic receptor agonist guanfacine but not lofexidine may reduce certain withdrawal symptoms such as irritability and sleep disruption but does not improve relapse to cannabis (Table 2). The side effects of lofexidine and poor tolerability limit its potential as a candidate for therapeutics. Other α2-adrenergic agonists alone or in combination with the CB1 receptor agonists may be further explored for cannabis abstinence, as guanfacine was well tolerated without increased side effects.
Drugs on the Horizon and Other Targets
N-Acetylcysteine (NAC) is a drug used for acetaminophen overdose, as a mucolytic, and has anti-inflammatory and antioxidant properties [140]. N-Acetylcysteine is able to stimulate the metabotropic glutamate receptors that are down-regulated with chronic drug use [141]. N-Acetylcysteine reduced the desire to use cocaine and cue-reactivity in cocaine-dependent adults [142] and has been shown to increase odds of abstinence in cannabis-dependent adolescents [63]. In animal models injected with propionic acid (known to cause adverse effects on the CNS and serotonin system), NAC has shown protective properties against oxidative stress and improved levels of serotonin [143]. N-Acetylcysteine has been explored as a potential treatment among adolescents and adults for CUD (Table 1). In the adolescent cohort, 41% of treatment-seeking participants receiving NAC (600 mg BID; 190/464) achieved abstinence compared to 27% placebo (126/464) [63]; however, a follow-up study in adults did not replicate these findings [68]. As such, the effects of NAC to reduce cannabis use seems to be age dependent and may rely on a variety of factors.
Cyclooxygenase-2 (COX-2) is an enzyme that is involved in the endocannabinoid metabolic pathway and oxygenates anandamide and 2-arachidonoylglycerol (2-AG) [144]. Cyclooxygenase-2 inhibitors have been shown to reduce withdrawal symptoms in alcohol-dependent mice and have been explored in humans [145]. Nontreatment-seeking participants receiving celecoxib (200 mg BID), a COX-2–selective inhibitor and nonsteroidal anti-inflammatory drug FDA-approved for osteoarthritis and rheumatoid arthritis, did not exhibit reduction in cannabis relapse, endocannabinoid levels, and increased cannabis craving compared to placebo [53]. Modulating the endocannabinoid via celecoxib appears ineffective.
Other targets have been explored as treatments for CUD, such as oxytocin and norepinephrine modulators (Table 1). In a cannabis-dependent sample that administered intranasal oxytocin (40 IUs) followed by a stress task, oxytocin reduced cannabis craving, suggesting oxytocin may be useful in real-world stressful scenarios that could trigger urge to use cannabis [146]. In a pilot study enrolling a treatment-seeking sample, intranasal oxytocin (40 IUs) paired with motivational enhancement therapy reduced the amount of cannabis used daily and the number of occasions used per day when compared to placebo [76]. Follow-up studies with larger sample sizes are necessary to probe additional effects of withdrawal, craving, and abstinence. Atomoxetine inhibits the reuptake of norepinephrine and is FDA-approved for ADHD [136]. A treatment-seeking sample with ADHD receiving atomoxetine (up to 100 mg) experienced improved ADHD symptoms but no change in cannabis use compared to placebo, suggesting limited potential for this pharmacotherapeutic strategy [75].
Finally, additional pharmacological strategies that use combinations of allosteric modulators may be promising for CUD with limited abuse liability as compared to orthostatic ligands. These allosteric modulators have the potential to be used with or without exogenous ligands. For example, the positive allosteric modulator Org27569 increases CB1 receptor signaling when used together with agonist in vivo [147].
Considering Priority Populations in the Exploration of Pharmacotherapies for CUD
As cannabis laws have become more permissive across the USA, cannabis use has increased among demographic subgroups traditionally believed to be at lower risk for cannabis initiation, use, and development of CUD. Among females, rates of monthly and daily cannabis use and CUD have increased [148]. Based on preclinical findings, there are clear sex-dependent effects of CB1 receptor agonists that may translate into enhanced vulnerability to CUD among females; for example, in preclinical studies, females exhibit faster acquisition of CB1 receptor agonist self-administration and maintain higher rates of responding for these agonists compared to males [149]. Similarly, under controlled human drug administration procedures, females exhibit greater misuse-related subjective drug effects associated with oral and inhaled THC compared to males [150, 151]. Females also appear to present with more severe cannabis withdrawal symptoms relative to males [152], which may increase risk for relapse among newly abstinent treatment seekers [153]. These effects demonstrate differences between males and females that are likely to be clinically significant when addressing potential pharmacotherapies for CUD. Very few studies probing potential pharmacotherapies have been powered to assessed sex-dependent effects (Table 1), although some preliminary analyses confirm that treatment outcomes can differ between the sexes pointing to the importance of designing studies to address pharmacotherapy efficacy in both males and females [66, 67].
Adolescents are another priority population of consideration in respect to the outcome of CUD. Major developmental changes involving CB1 receptors occur during adolescence that may impact cognitive functioning later in life, particularly increasing vulnerability for psychotic-like symptoms [154]. Additionally, the risk of developing a CUD increases if initiation occurred before age 18 [155], with the odds decreasing with each year of delayed cannabis use [156], highlighting the importance of intervention for this age group. Few studies have investigated pharmacotherapies for CUD in adolescents and emerging adults (although see [63]).
One group exhibiting the greatest increase in cannabis use is the aging population [157]. While few data are available related to the effects of cannabis use in older populations, preliminary findings indicate that older population vary in their responses to cannabis in ways that may be clinically meaningful when considering identifying and treatment CUD in this population. For example, recent data point to potentially lower rates of tolerance to the impairing effects of cannabis in older populations relative to what is observed in younger populations [158, 159]. It is unclear how this effect may impact development of CUD in this population but this example highlights the importance of establishing and characterizing the trajectory of cannabis initiation to CUD among older adults and exploring pharmacotherapies tailored to this population.
Rates of cannabis initiation, cannabis use, and CUD are higher among racial, ethnic, and sexual minoritized populations (i.e., [160–162]). As such, randomized controlled trials (RCTs) probing pharmacotherapies for CUD should be optimized to detect treatment outcomes among these groups. However, similar to RCTs of pharmacotherapies for CUD that have evaluated treatment outcomes according to sex, few studies have assessed outcomes according to race and ethnicity and none, to our knowledge, have assessed treatment outcomes in sexual minority populations [163].
Discussion
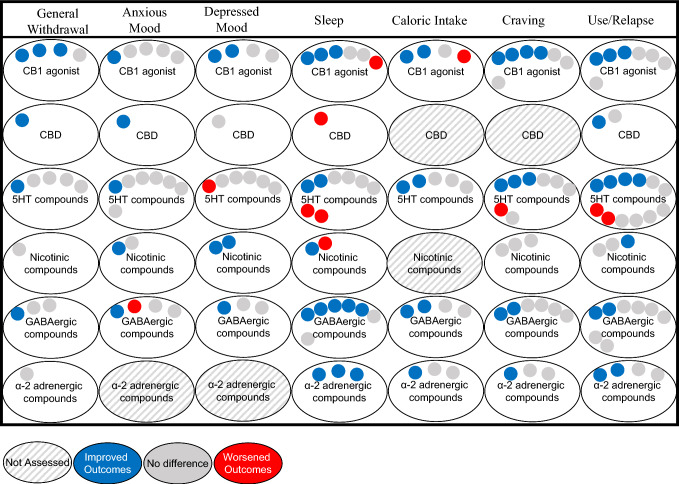
As CUD increases in prevalence, so does the need for effective pharmacotherapies. Barriers to cessation of cannabis use include relieving both craving for cannabis and withdrawal symptoms; however, effective treatments for CUD will ultimately need to result in cessation of cannabis consumption regardless of whether treatments reduce craving or withdrawal. This review evaluated pharmacotherapies based on the endogenous systems targeted by the compounds. The most effective treatments for decreasing cannabis withdrawal, craving, and relapse are agents that act at the CB1 receptor (Fig. 1). There is also limited but promising evidence on fatty acid amide hydrolase inhibitors for decreasing withdrawal and relapse. Mixed findings of serotonergic compounds as well as other less promising agents such as nicotinic compounds and GABAergic compounds were evaluated (Fig. 1). Agents with limited studies or limited outcome measures include opioidergic compounds, α2-adrenergic compounds, and drugs on the horizon such as oxytocin. Although agents acting directly on the endocannabinoid system appear to provide the most promising outcomes, continued research into receptor systems and pharmacological targets beyond the endocannabinoid system is essential as the field progresses to learn more about how these targets contribute to the constellation of CUD symptoms. Furthermore, mixed and limited findings from non-cannabinoid agents may not reflect lack of efficacy but may be due to a variety of factors such as the doses of the medications studied, differences in eligibility criteria across studies, and participant compliance in taking the study medication.
Fig. 1.
Visual summary of outcome measures categorized by mechanism of action in published studies. Drug categories with only one study are omitted from this visual, such as FAAH inhibitors, opioidergic compounds, and drugs on the horizon. 5HT serotonin, CB1 cannabinoid receptor 1, CBD cannabidiol, FAAH fatty acid amide hydrolase
A major gap in the literature on CUD pharmacotherapies exists in the populations being sampled. As clinical trials become more inclusive with regard to demographics such as sex, race/ethnicity, and age, disparities are revealed in CUD treatment outcomes. As new pharmacotherapies are tested in clinical trials, it is critical to enroll representative samples of the population, as differences in outcomes by sex for some drugs have been noted. Evaluating these nuances in statistical analyses has the promise to elucidate contradictory findings, unknown mediators, and provide clarity on the nature of CUD.
Conclusion
This review provides an updated synthesis of findings from over 40 clinical placebo-controlled trials on treatment-seeking and nontreatment-seeking samples in context of neurological systems and receptors. While some pharmacological agents seem promising for treating the symptoms associated with CUD, many questions remain to be answered by future experiments. The current literature presents CB1 agonists as emerging targets [39–42, 55–57, 86], but data points on studies examining cannabidiol [43, 58], FAAH inhibitors [99], and opioidergic targets [48] remain scant. Understanding the landscape of possible treatments for CUD for the development of effective treatments is important as rates of cannabis use increase and legalization changes. The presented findings offer insight into the promising possibility of treatments that modulate CB1 receptors.
Acknowledgements
This work was supported by the Intramural Research Program, US National Institute on Drug Abuse (DA047296, DA047296S1 and R25DA050723), the Semel Charitable Foundation, the Center for Medicinal Cannabis Research (CMCR), UCLA Shirley and Stefan Hatos Center for Neuropharmacology, and UCLA Training Program on the Translational Neuroscience of Drug Abuse (TNDA) predoctoral T32 funding.
Declarations
Funding
Outside of this work, ZDC reports receiving study drug from Canopy Growth Corp and True Terpenes, and study-related materials from Storz & Bickel. She served as a scientific consultant for Canopy Growth Corporation in 2021 and on the scientific advisory board for FSD Pharma in 2020. ZDC’s research is funded by grants from the National Institute on Drug Abuse, National Center for Complementary and Integrative Health, California Department of Cannabis Control, Center for Medicinal Cannabis Research, and the California Highway Patrol.
Conflict of interest
The authors declare that they have no competing interests or disclosures with this publication.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author’s contributions
MA: Draft preparation, writing, and review; BAH Draft preparation and writing; CMC reviewing and editing of final submission; ZDC: reviewing, editing, and preparation of final submission.
Author’s approval
All authors have read and approved the final submitted manuscript and agree to be accountable for the work.
References
- 1.Substance Abuse and Mental Health Services Administration. In: SAMHSA Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2019 and Quarters 1 and 4, 2020.
- 2.Patel J, Marwaha R. Cannabis use disorder. Treasure Island: StatPearls Publishing; Updated; 2022. [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2021 National Survey on Drug Use and Health (HHS Publication No. PEP22-07-01-005, NSDUH Series H-57). Center for behavioral health statistics and quality, substance abuse and mental health services administration. 2022.
- 4.National Academies of Sciences Engineering and Medicine. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. 2017. 10.17226/24625 [PubMed]
- 5.Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474–83. 10.1001/jama.2015.6199. [DOI] [PubMed] [Google Scholar]
- 6.Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9(6):506–21. 10.1016/j.jpain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musty RE, Rossi R. Effects of smoked cannabis and oral δ9-tetrahydrocannabinol on nausea and emesis after cancer chemotherapy. J Cannabis Ther. 2001;1(1):29–56. 10.1300/J175v01n01_03. [Google Scholar]
- 8.The American Cancer Society medical and editorial content team. Marijuana and Cancer. 2022 August 3 2022. https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/complementary-and-integrative-medicine/marijuana-and-cancer.html. Accessed 01 Dec 2023.
- 9.Babor TF, Carroll KM, Christiansen K, Donaldson J, Herrell JM, Kadden RR, et al. Brief treatments for cannabis dependence: findings from a randomized multisite trial. J Consult Clin Psychol. 2004;72(3):455–66. [DOI] [PubMed] [Google Scholar]
- 10.Schuster RM, Hanly A, Gilman J, Budney A, Vandrey R, Evins AE. A contingency management method for 30-days abstinence in non-treatment seeking young adult cannabis users. Drug Alcohol Depend. 2016;1(167):199–206. 10.1016/j.drugalcdep.2016.08.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes JR, Naud S, Budney AJ, Fingar JR, Callas PW. Attempts to stop or reduce daily cannabis use: an intensive natural history study. Psychol Addict Behav: J Soc Psychol Addict Behav. 2016;30(3):389–97. 10.1037/adb0000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budney AJ, Sofis MJ, Borodovsky JT. An update on cannabis use disorder with comment on the impact of policy related to therapeutic and recreational cannabis use. Eur Arch Psychiatry Clin Neurosci. 2019;269(1):73–86. 10.1007/s00406-018-0976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budney AJ, Vandrey RG, Stanger C. Pharmacological and psychosocial interventions for cannabis use disorders. Braz J Psychiatry. 2010;32(Suppl 1):S46-55. [PMC free article] [PubMed] [Google Scholar]
- 14.Budney AJ, Moore BA, Rocha HL, Higgins ST. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J Consult Clin Psychol. 2006;74(2):307–16. 10.1037/0022-006x.4.2.307. [DOI] [PubMed] [Google Scholar]
- 15.Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict Behav. 2007;32(6):1220–36. 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 17.Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology. 2018;43(1):34–51. 10.1038/npp.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–60. 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet U, Preuss UW. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9–37. 10.2147/SAR.S109576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393–402. 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- 21.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;141(4):395–404. 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 22.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology. 1999;141(4):385–94. 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- 23.Livne O, Shmulewitz D, Lev-Ran S, Hasin DS. DSM-5 cannabis withdrawal syndrome: demographic and clinical correlates in US adults. Drug Alcohol Depend. 2019;195:170–7. 10.1016/j.drugalcdep.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahji A, Stephenson C, Tyo R, Hawken ER, Seitz DP. Prevalence of cannabis withdrawal symptoms among people with regular or dependent use of cannabinoids: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(4):e202370–e202370. 10.1001/jamanetworkopen.2020.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, et al. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006;15(1):8–14. 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- 26.Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL, et al. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 2010;111(1–2):120–7. 10.1016/j.drugalcdep.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson PB. Phytocannabinoid pharmacology: medicinal properties of Cannabis sativa constituents aside from the “Big Two.” J Nat Prod. 2021;84(1):142–60. 10.1021/acs.jnatprod.0c00965. [DOI] [PubMed] [Google Scholar]
- 28.Cooper ZD, Haney M. Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence. Int Rev Psychiatry. 2009;21(2):104–12. 10.1080/09540260902782752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper ZD, Haney M. Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addict Biol. 2008;13(2):188–95. 10.1111/j.1369-1600.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br J Pharmacol. 2010;161(1):103–12. 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17(6):642–9. 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman BJ, McRae-Clark AL. Neurotransmitter and neuropeptide targets for cannabis use disorder treatment. In: Montoya ID, Weiss SRB, editors. Cannabis use disorders. Cham: Springer International Publishing; 2019. p. 207–11. [Google Scholar]
- 33.Cerdá M, Mauro C, Hamilton A, Levy NS, Santaella-Tenorio J, Hasin D, et al. Association between recreational marijuana legalization in the United States and changes in marijuana use and cannabis use disorder from 2008 to 2016. JAMA Psychiat. 2020;77(2):165–71. 10.1001/jamapsychiatry.2019.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal A, Lynskey MT. Candidate genes for cannabis use disorders: findings, challenges and directions. Addiction. 2009;104(4):518–32. 10.1111/j.1360-0443.2009.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuttler C, Mischley LK, Sexton M. Sex differences in cannabis use and effects: a cross-sectional survey of cannabis users. Cannabis Cannabinoid Res. 2016;1(1):166–75. 10.1089/can.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knaappila N, Marttunen M, Fröjd S, Lindberg N, Kaltiala R. Changes in cannabis use according to socioeconomic status among Finnish adolescents from 2000 to 2015. J Cannabis Res. 2020;2(1):44. 10.1186/s42238-020-00052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodgins DC, Stea JN. Insights from individuals successfully recovered from cannabis use disorder: natural versus treatment-assisted recoveries and abstinent versus moderation outcomes. Addict Sci Clin Pract. 2018;13(1):16. 10.1186/s13722-018-0118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol Psychiatry. 2016;79(7):613–9. 10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29(1):158–70. 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- 40.Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86(1):22–9. 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197(1):157–68. 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology. 2013;38(8):1557–65. 10.1038/npp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. 2016;41(8):1974–82. 10.1038/npp.2015.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haney M, Hart CL, Ward AS, Foltin RW. Nefazodone decreases anxiety during marijuana withdrawal in humans. Psychopharmacology. 2003;165(2):157–65. 10.1007/s00213-002-1210-3. [DOI] [PubMed] [Google Scholar]
- 45.Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Cooper ZD, et al. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2010;211(2):233–44. 10.1007/s00213-010-1888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M. A human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addict Biol. 2013;18(6):993–1002. 10.1111/j.1369-1600.2012.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arout CA, Cooper ZD, Reed SC, Foltin RW, Comer SD, Levin FR, et al. 5HT-2C agonist lorcaserin decreases cannabis self-administration in daily cannabis smokers. Addict Biol. 2021;26(4):e12993. 10.1111/adb.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haney M, Ramesh D, Glass A, Pavlicova M, Bedi G, Cooper ZD. Naltrexone maintenance decreases cannabis self-administration and subjective effects in daily cannabis smokers. Neuropsychopharmacology. 2015;40(11):2489–98. 10.1038/npp.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Varenicline and nabilone in tobacco and cannabis co-users: effects on tobacco abstinence, withdrawal and a laboratory model of cannabis relapse. Addict Biol. 2019;24(4):765–76. 10.1111/adb.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117(1):38–44. 10.1016/j.drugalcdep.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology. 2016;233(13):2469–78. 10.1007/s00213-016-4298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haney M, Cooper ZD, Bedi G, Herrmann E, Comer SD, Reed SC, et al. Guanfacine decreases symptoms of cannabis withdrawal in daily cannabis smokers. Addict Biol. 2019;24(4):707–16. 10.1111/adb.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haney M, Bedi G, Cooper ZD, Herrmann ES, Reed SC, Foltin RW, et al. Impact of cyclooxygenase-2 inhibition on cannabis withdrawal and circulating endocannabinoids in daily cannabis smokers. Addict Biol. 2022;27(4):e13183. 10.1111/adb.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haney M, Vallée M, Fabre S, Collins Reed S, Zanese M, Campistron G, et al. Signaling-specific inhibition of the CB1 receptor for cannabis use disorder: phase 1 and phase 2a randomized trials. Nat Med. 2023. 10.1038/s41591-023-02381-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116(1–3):142–50. 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiat. 2014;71(3):281–91. 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- 57.Lintzeris N, Bhardwaj A, Mills L, Dunlop A, Copeland J, McGregor I, et al. Nabiximols for the treatment of cannabis dependence: a randomized clinical trial. JAMA Intern Med. 2019;179(9):1242–53. 10.1001/jamainternmed.2019.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freeman TP, Hindocha C, Baio G, Shaban ND, Thomas EM, Astbury D, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. 2020;7(10):865–74. 10.1016/S2215-0366(20)30290-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology. 2001;155(2):171–9. 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- 60.McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, et al. A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug Alcohol Depend. 2009;105(1–2):132–8. 10.1016/j.drugalcdep.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnston J, Lintzeris N, Allsop DJ, Suraev A, Booth J, Carson DS, et al. Lithium carbonate in the management of cannabis withdrawal: a randomized placebo-controlled trial in an inpatient setting. Psychopharmacology. 2014;231(24):4623–36. 10.1007/s00213-014-3611-5. [DOI] [PubMed] [Google Scholar]
- 62.Penetar DM, Looby AR, Ryan ET, Maywalt MA, Lukas SE. Bupropion reduces some of the symptoms of marihuana withdrawal in chronic marihuana users: a pilot study. Subst Abuse. 2012;6:63–71. 10.4137/sart.S9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805–12. 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levin FR, Mariani J, Brooks DJ, Pavlicova M, Nunes EV, Agosti V, et al. A randomized double-blind, placebo-controlled trial of venlafaxine-extended release for co-occurring cannabis dependence and depressive disorders. Addiction. 2013;108(6):1084–94. 10.1111/add.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinstein AM, Miller H, Bluvstein I, Rapoport E, Schreiber S, Bar-Hamburger R, et al. Treatment of cannabis dependence using escitalopram in combination with cognitive-behavior therapy: a double-blind placebo-controlled study. Am J Drug Alcohol Abuse. 2014;40(1):16–22. 10.3109/00952990.2013.819362. [DOI] [PubMed] [Google Scholar]
- 66.McRae-Clark AL, Baker NL, Gray KM, Killeen TK, Wagner AM, Brady KT, et al. Buspirone treatment of cannabis dependence: a randomized, placebo-controlled trial. Drug Alcohol Depend. 2015;1(156):29–37. 10.1016/j.drugalcdep.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McRae-Clark AL, Baker NL, Gray KM, Killeen T, Hartwell KJ, Simonian SJ. Vilazodone for cannabis dependence: a randomized, controlled pilot trial. Am J Addict. 2016;25(1):69–75. 10.1111/ajad.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gray KM, Sonne SC, McClure EA, Ghitza UE, Matthews AG, McRae-Clark AL, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;1(177):249–57. 10.1016/j.drugalcdep.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mariani JJ, Pavlicova M, Jean Choi C, Basaraba C, Carpenter KM, Mahony AL, et al. Quetiapine treatment for cannabis use disorder. Drug Alcohol Depend. 2021;1(218): 108366. 10.1016/j.drugalcdep.2020.108366. [DOI] [PubMed] [Google Scholar]
- 70.Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, et al. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict. 2004;13(1):21–32. 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- 71.Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689–98. 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miranda R Jr, Treloar H, Blanchard A, Justus A, Monti PM, Chun T, et al. Topiramate and motivational enhancement therapy for cannabis use among youth: a randomized placebo-controlled pilot study. Addict Biol. 2017;22(3):779–90. 10.1111/adb.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee DC, Schlienz NJ, Herrman ES, Martin EL, Leoutsakos J, Budney AJ, et al. Randomized controlled trial of zolpidem as a pharmacotherapy for cannabis use disorder. J Subst Use Addict Treat. 2023. 10.1016/j.josat.2023.209180. [DOI] [PubMed] [Google Scholar]
- 74.Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, et al. Dronabinol and lofexidine for cannabis use disorder: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2016;1(159):53–60. 10.1016/j.drugalcdep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, White KG, Brady KT. A placebo-controlled trial of atomoxetine in marijuana-dependent individuals with attention deficit hyperactivity disorder. Am J Addict. 2010;19(6):481–9. 10.1111/j.1521-0391.2010.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sherman BJ, Baker NL, McRae-Clark AL. Effect of oxytocin pretreatment on cannabis outcomes in a brief motivational intervention. Psychiatry Res. 2017;249:318–20. 10.1016/j.psychres.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McRae-Clark AL, Gray KM, Baker NL, Sherman BJ, Squeglia L, Sahlem GL, et al. Varenicline as a treatment for cannabis use disorder: a placebo-controlled pilot trial. Drug Alcohol Depend. 2021;229(Pt B): 109111. 10.1016/j.drugalcdep.2021.109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spiller KJ, Bi GH, He Y, Galaj E, Gardner EL, Xi ZX. Cannabinoid CB(1) and CB(2) receptor mechanisms underlie cannabis reward and aversion in rats. Br J Pharmacol. 2019;176(9):1268–81. 10.1111/bph.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3(11):1073–4. 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- 80.Schindler CW, Redhi GH, Vemuri K, Makriyannis A, Le Foll B, Bergman J, et al. Blockade of nicotine and cannabinoid reinforcement and relapse by a cannabinoid CB1-receptor neutral antagonist AM4113 and inverse agonist rimonabant in squirrel monkeys. Neuropsychopharmacology. 2016;41(9):2283–93. 10.1038/npp.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Braida D, Iosue S, Pegorini S, Sala M. Δ9-Tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004;506(1):63–9. 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 82.Freels TG, Baxter-Potter LN, Lugo JM, Glodosky NC, Wright HR, Baglot SL, et al. Vaporized cannabis extracts have reinforcing properties and support conditioned drug-seeking behavior in rats. J Neurosci. 2020;40(9):1897–908. 10.1523/jneurosci.2416-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1998;285(3):1150–6. [PubMed] [Google Scholar]
- 84.Lichtman AH, Fisher J, Martin BR. Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav. 2001;69(1–2):181–8. 10.1016/s0091-3057(01)00514-7. [DOI] [PubMed] [Google Scholar]
- 85.Tai S, Nikas SP, Shukla VG, Vemuri K, Makriyannis A, Järbe TU. Cannabinoid withdrawal in mice: inverse agonist vs neutral antagonist. Psychopharmacology. 2015;232(15):2751–61. 10.1007/s00213-015-3907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hill KP, Palastro MD, Gruber SA, Fitzmaurice GM, Greenfield SF, Lukas SE, et al. Nabilone pharmacotherapy for cannabis dependence: A randomized, controlled pilot study. Am J Addict. 2017;26(8):795–801. 10.1111/ajad.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lintzeris N, Mills L, Dunlop A, Copeland J, McGregor I, Bruno R, et al. Cannabis use in patients 3 months after ceasing nabiximols for the treatment of cannabis dependence: Results from a placebo-controlled randomised trial. Drug Alcohol Depend. 2020;215:108220. 10.1016/j.drugalcdep.2020.108220. [DOI] [PubMed] [Google Scholar]
- 88.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;6(2):Cd002207. 10.1002/14651858.CD002207.pub4. [DOI] [PubMed] [Google Scholar]
- 89.Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58(4):322–8. 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- 90.Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, et al. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology. 2007;194(4):505–15. 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johansson K, Neovius K, DeSantis SM, Rössner S, Neovius M. Discontinuation due to adverse events in randomized trials of orlistat, sibutramine and rimonabant: a meta-analysis. Obes Rev. 2009;10(5):564–75. 10.1111/j.1467-789X.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen T, Thomas BF, Zhang Y. Overcoming the psychiatric side effects of the cannabinoid CB1 receptor antagonists: current approaches for therapeutics development. Curr Top Med Chem. 2019;19(16):1418–35. 10.2174/1568026619666190708164841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vallée M, Vitiello S, Bellocchio L, Hébert-Chatelain E, Monlezun S, Martin-Garcia E, et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343(6166):94–8. 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McKinney MK, Cravatt BF. Structure and function of fatty acid amide hydrolase. Annu Rev Biochem. 2005;74(1):411–32. 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- 95.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–7. 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 96.Boileau I, Mansouri E, Williams B, Le Foll B, Rusjan P, Mizrahi R, et al. Fatty acid amide hydrolase binding in brain of cannabis users: imaging with the novel radiotracer [(11)C]CURB. Biol Psychiatry. 2016;80(9):691–701. 10.1016/j.biopsych.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schlosburg JE, Carlson BL, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, et al. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 2009;11(2):342–52. 10.1208/s12248-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morgan CJA, Page E, Schaefer C, Chatten K, Manocha A, Gulati S, et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry. 2013;202(5):381–2. 10.1192/bjp.bp.112.121178. [DOI] [PubMed] [Google Scholar]
- 99.D’Souza DC, Cortes-Briones J, Creatura G, Bluez G, Thurnauer H, Deaso E, et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry. 2019;6(1):35–45. 10.1016/s2215-0366(18)30427-9. [DOI] [PubMed] [Google Scholar]
- 100.D'Souza DC. A Phase 2B, 8-week, Randomized, Double-Blind, Placebo-Controlled, Parallel Study to Evaluate Efficacy, Safety and Tolerability of the Fatty Acid Amide Hydrolase (FAAH) Inhibitor PF-04457845 in Adults With DSM5 Current Cannabis Use Disorder (CUD). December 29 2017. https://clinicaltrials.gov/ct2/show/study/NCT03386487?term=PF-04457845&draw=2&rank=6.
- 101.Watanabe K, Kayano Y, Matsunaga T, Yamamoto I, Yoshimura H. Inhibition of anandamide amidase activity in mouse brain microsomes by cannabinoids. Biol Pharm Bull. 1996;19(8):1109–11. 10.1248/bpb.19.1109. [DOI] [PubMed] [Google Scholar]
- 102.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–805. 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613–23. 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pretzsch CM, Freyberg J, Voinescu B, Lythgoe D, Horder J, Mendez MA, et al. Effects of cannabidiol on brain excitation and inhibition systems; a randomised placebo-controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacology. 2019;44(8):1398–405. 10.1038/s41386-019-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30(8):1037–43. 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- 106.Gonzalez-Cuevas G, Martin-Fardon R, Kerr TM, Stouffer DG, Parsons LH, Hammell DC, et al. Unique treatment potential of cannabidiol for the prevention of relapse to drug use: preclinical proof of principle. Neuropsychopharmacology. 2018;43(10):2036–45. 10.1038/s41386-018-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maccioni P, Bratzu J, Carai MAM, Colombo G, Gessa GL. Reducing effect of cannabidiol on alcohol self-administration in sardinian alcohol-preferring rats. Cannabis Cannabinoid Res. 2022;7(2):161–9. 10.1089/can.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morgan CJA, Das RK, Joye A, Curran HV, Kamboj SK. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict Behav. 2013;38(9):2433–6. 10.1016/j.addbeh.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 109.Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176(11):911–22. 10.1176/appi.ajp.2019.18101191. [DOI] [PubMed] [Google Scholar]
- 110.Bambico FR, Nguyen N-T, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis. 2010;37(3):641–55. 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 111.Berthoux C, Hamieh AM, Rogliardo A, Doucet EL, Coudert C, Ango F, et al. Early 5-HT6 receptor blockade prevents symptom onset in a model of adolescent cannabis abuse. EMBO Mol Med. 2020;12(5):e10605. 10.15252/emmm.201910605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haj-Dahmane S, Shen RY. Modulation of the serotonin system by endocannabinoid signaling. Neuropharmacology. 2011;61(3):414–20. 10.1016/j.neuropharm.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Collins GT, Gerak LR, France CP. The behavioral pharmacology and therapeutic potential of lorcaserin for substance use disorders. Neuropharmacology. 2018;142:63–71. 10.1016/j.neuropharm.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, et al. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology. 2012;37(5):1177–91. 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Collins GT, Gerak LR, Javors MA, France CP. Lorcaserin reduces the discriminative stimulus and reinforcing effects of cocaine in Rhesus Monkeys. J Pharmacol Exp Ther. 2016;356(1):85–95. 10.1124/jpet.115.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kohut SJ, Bergman J. Lorcaserin decreases the reinforcing effects of heroin, but not food, in rhesus monkeys. Eur J Pharmacol. 2018;5(840):28–32. 10.1016/j.ejphar.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ. The 5-HT(2C) receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology. 2016;101:237–45. 10.1016/j.neuropharm.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 118.Howell LL, Cunningham KA. Serotonin 5-HT<sub>2</sub> receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67(1):176–97. 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. Am J Addict. 2009;18(1):53–64. 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davis R, Wilde MI. Mirtazapine: a review of its pharmacology and therapeutic potential in the management of major depression. CNS Drugs. 1996;5(5):389–402. 10.2165/00023210-199605050-00007. [DOI] [PubMed] [Google Scholar]
- 121.Liappas J, Paparrigopoulos T, Tzavellas E, Rabavilas A. Mirtazapine and venlafaxine in the management of collateral psychopathology during alcohol detoxification. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(1):55–60. 10.1016/j.pnpbp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 122.Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22(3):1146–54. 10.1523/jneurosci.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Δ9-tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology. 2004;173(1):186–94. 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- 124.Cooper ZD, Haney M. Opioid antagonism enhances marijuana’s effects in heavy marijuana smokers. Psychopharmacology. 2010;211(2):141–8. 10.1007/s00213-010-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction. 2012;107(7):1221–33. 10.1111/j.1360-0443.2012.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hindocha C, Shaban ND, Freeman TP, Das RK, Gale G, Schafer G, et al. Associations between cigarette smoking and cannabis dependence: a longitudinal study of young cannabis users in the United Kingdom. Drug Alcohol Depend. 2015;1(148):165–71. 10.1016/j.drugalcdep.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, et al. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol Psychiatry. 2013;73(3):242–8. 10.1016/j.biopsych.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ng Cheong Ton JM, Gerhardt GA, Friedemann M, Etgen AM, Rose GM, Sharpless NS, et al. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res. 1988;451(1–2):59–68. 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- 129.Bossong MG, van Berckel BNM, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Δ9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34(3):759–66. 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- 130.Szabo B, Siemes S, Wallmichrath I. Short communication inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15(12):2057–61. 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- 131.Shollenbarger SG, Price J, Wieser J, Lisdahl K. Impact of cannabis use on prefrontal and parietal cortex gyrification and surface area in adolescents and emerging adults. Dev Cogn Neurosci. 2015;16:46–53. 10.1016/j.dcn.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zamberletti E, Beggiato S, Steardo L, Prini P, Antonelli T, Ferraro L, et al. Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiol Dis. 2014;63:35–47. 10.1016/j.nbd.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 133.Lile JA, Kelly TH, Hays LR. Separate and combined effects of the GABA reuptake inhibitor tiagabine and Δ9-THC in humans discriminating Δ9-THC. Drug Alcohol Depend. 2012;122(1–2):61–9. 10.1016/j.drugalcdep.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen L, Xie JX, Fung KS, Yung WH. Zolpidem modulates GABA(A) receptor function in subthalamic nucleus. Neurosci Res. 2007;58(1):77–85. 10.1016/j.neures.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 135.Mason BJ. Gabapentin Treatment of Cannabis Dependence. 2017. https://clinicaltrials.gov/study/NCT00395044?term=Mason&intr=gabapentin&aggFilters=results:with&rank=2.
- 136.US Food & Drug Administration. Drugs@FDA: FDA-Approved Drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed 12 June 2024.
- 137.Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;2016(5):Cd002024. 10.1002/14651858.CD002024.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Giovannitti JA Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62(1):31–9. 10.2344/0003-3006-62.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cathel AM, Reyes BA, Wang Q, Palma J, Mackie K, Van Bockstaele EJ, et al. Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex. Eur J Neurosci. 2014;40(8):3202–14. 10.1111/ejn.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tenório M, Graciliano NG, Moura FA, Oliveira ACM, Goulart MOF. N-Acetylcysteine (NAC): impacts on human health. Antioxidants (Basel). 2021. 10.3390/antiox10060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.D’Souza MS. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci. 2015. 10.3389/fnins.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Steven D, LaRowe PD, Hugh Myrick MD, Sarra Hedden MS, Pascale Mardikian MD, Michael Saladin PD, Aimee McRae PD, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164(7):1115–7. 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 143.Aldbass AM, Bhat RS, El-Ansary A. Protective and therapeutic potency of N-acetyl-cysteine on propionic acid-induced biochemical autistic features in rats. J Neuroinflamm. 2013;10(1):837. 10.1186/1742-2094-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kozak KR, Prusakiewicz JJ, Marnett LJ. Oxidative metabolism of endocannabinoids by COX-2. Curr Pharm Des. 2004;10(6):659–67. 10.2174/1381612043453081. [DOI] [PubMed] [Google Scholar]
- 145.Dhir A, Naidu PS, Kulkarni SK. Protective effect of cyclooxygenase-2 (COX-2) inhibitors but not non-selective cyclooxygenase (COX)-inhibitors on ethanol withdrawal-induced behavioural changes. Addict Biol. 2005;10(4):329–35. 10.1080/13556210500352964. [DOI] [PubMed] [Google Scholar]
- 146.McRae-Clark AL, Baker NL, Maria MM, Brady KT. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology. 2013;228(4):623–31. 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ahn KH, Mahmoud MM, Kendall DA. Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J Biol Chem. 2012;287(15):12070–82. 10.1074/jbc.M111.316463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services. 2020 National Survey on Drug Use and Health: Women. In: National Survey on Drug Use and Health; 2022.
- 149.Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol. 2007;152(5):795–804. 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fogel JS, Kelly TH, Westgate PM, Lile JA. Sex differences in the subjective effects of oral Δ(9)-THC in cannabis users. Pharmacol Biochem Behav. 2017;152:44–51. 10.1016/j.pbb.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014;1(136):85–91. 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment-seeking cannabis users. Exp Clin Psychopharmacol. 2015;23(6):415–21. 10.1037/pha0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J, et al. Quantifying the clinical significance of cannabis withdrawal. PLoS One. 2012;7(9): e44864. 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol. 2010;160(3):511–22. 10.1111/j.1476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Winters KC, Lee CY. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug Alcohol Depend. 2008;92(1–3):239–47. 10.1016/j.drugalcdep.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Millar SR, Mongan D, Smyth BP, Perry IJ, Galvin B. Relationships between age at first substance use and persistence of cannabis use and cannabis use disorder. BMC Public Health. 2021;21(1):997. 10.1186/s12889-021-11023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lloyd SL, Striley CW. Marijuana use among adults 50 years or older in the 21st century. Gerontol Geriatr Med. 2018;4:2333721418781668. 10.1177/2333721418781668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Di Ciano P, Rajji TK, Hong L, Zhao S, Byrne P, Elzohairy Y, et al. Cannabis and driving in older adults. JAMA Netw Open. 2024;7(1):e2352233. 10.1001/jamanetworkopen.2023.52233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wiley JL, O’Connell MM, Tokarz ME, Wright MJ Jr. Pharmacological effects of acute and repeated administration of Delta(9)-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320(3):1097–105. 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]
- 160.Wu LT, Zhu H, Swartz MS. Trends in cannabis use disorders among racial/ethnic population groups in the United States. Drug Alcohol Depend. 2016;1(165):181–90. 10.1016/j.drugalcdep.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mauro PM, Philbin MM, Greene ER, Diaz JE, Askari MS, Martins SS. Daily cannabis use, cannabis use disorder, and any medical cannabis use among US adults: associations within racial, ethnic, and sexual minoritized identities in a changing policy context. Prev Med Rep. 2022;28: 101822. 10.1016/j.pmedr.2022.101822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Boyd CJ, Veliz PT, McCabe SE. Severity of DSM-5 cannabis use disorders in a nationally representative sample of sexual minorities. Subst Abus. 2020;41(2):191–5. 10.1080/08897077.2019.1621242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Montgomery L, Petry NM, Carroll KM. Moderating effects of race in clinical trial participation and outcomes among marijuana-dependent young adults. Drug and Alcohol Depend. 2012;126(3):333–9. 10.1016/j.drugalcdep.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]