FIGURE 4.
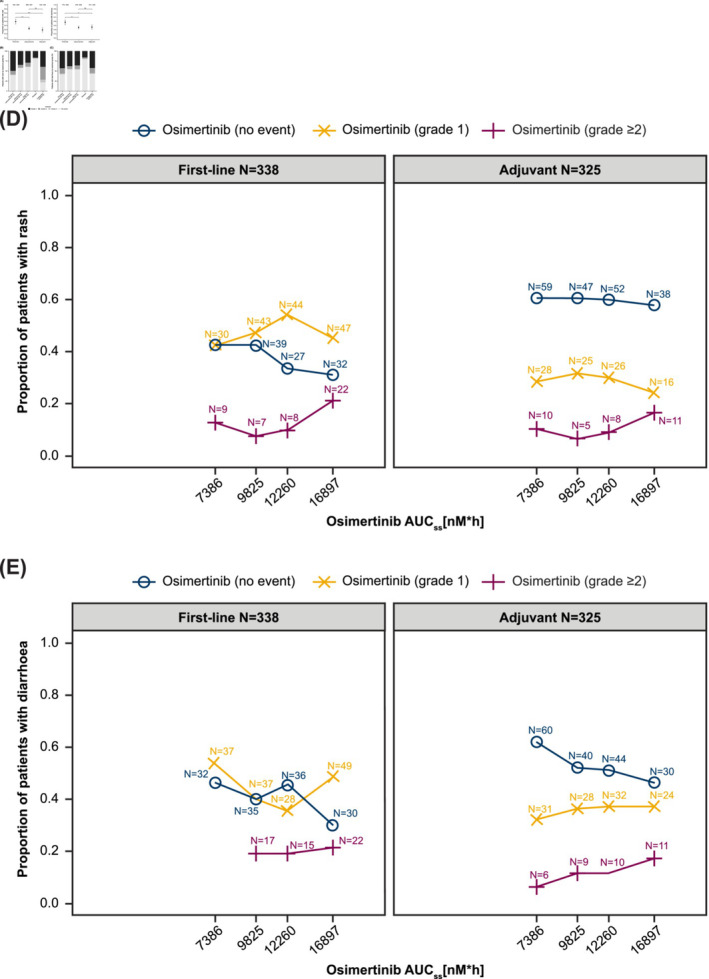
Descriptive analysis of rash and diarrhoea. Incidence of all‐grade rash and diarrhoea with osimertinib 80 mg across different lines of therapy (A). Distribution of maximum CTCAE grades of rash (B) and diarrhoea (C) occurring with osimertinib 80 mg, comparator EGFR‐TKI, and placebo. Proportion of CTCAE rash grades (D) and CTCAE diarrhoea grades (E) by osimertinib AUCss quartiles. AUCss, area under the curve at steady state; CTCAE, Common Terminology Criteria for Adverse Events; EGFR‐TKI, epidermal growth factor receptor‐tyrosine kinase inhibitor; ns, not significant.
