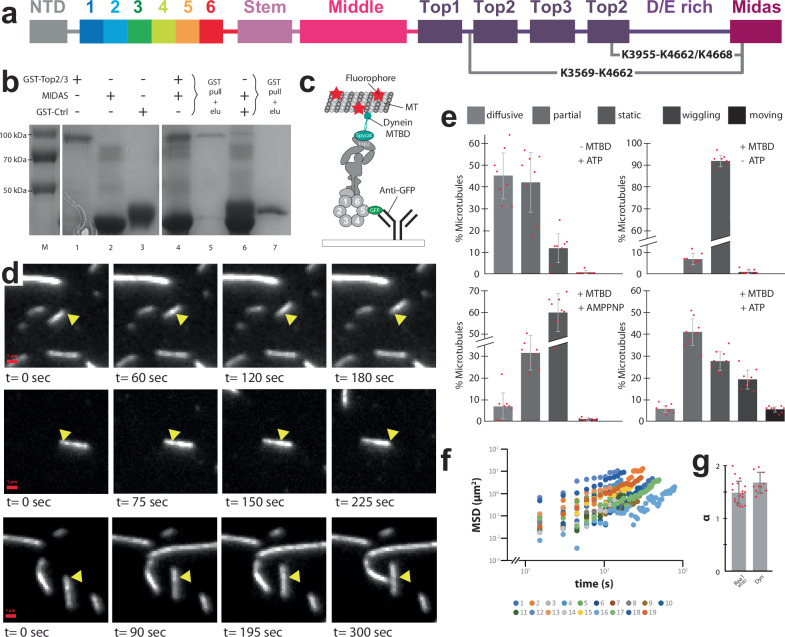
Fig. 5. The linker top interacts with the MIDAS domain and linker remodelling is a force producing event.
a Crosslinks supporting state 8 detected in the Rea1ΔAAA2H2α ATPγS data set. The K3569-K4662 and K3955-K4662/K4668 crosslinks suggest an interaction between the linker top2 domain and a conserved MIDAS domain loop. b GST pulldown experiments provide further support for the interaction between the MIDAS and linker top2/3 domains. The samples were run on a SDS gel and stained with Coomassie blue. The GST-linker top2/3 fragment pulls down the MIDAS domain (lane 5). M: marker, lanes 1, 2 and 3: purified linker top2/3 construct, MIDAS domain and GST control. Lane 4, 5: GST-linker top2/3 + MIDAS domain input and pulldown. Lane 6, 7: GST-control + MIDAS domain input and pulldown. The pulldown experiment was carried out once. c Microtubule gliding assays provide evidence that Rea1 linker remodelling produces force. The dynein microtubule binding domain (cyan) was fused to the linker top2 domain using the spycatcher/spytag approach. GFP was fused to AAA5. The construct was anchored to a cover slide via GFP-antibodies and fluorescently labelled microtubules we applied. Adapted from Sosnowski et al.17. Elife 7, 10.7554/eLife.39163 (2018) under a CC BY license: https://creativecommons.org/licenses/by/4.0/. d Movie frames of microtubule gliding events. Yellow arrow heads mark the microtubule position at the beginning of the movie (Supplementary Movies 3–5). The gliding events suggest that linker remodelling with respect to the AAA+ ring is able to produce mechanical force. e Statistical analysis of diffusive, partially bound, static, wiggling and moving microtubules under different conditions (n = 8). Moving microtubules are only observed in the presence of the dynein microtubule domain (MTBD) and ATP. f, g The microtubule gliding events result from directed movement. f Log mean squared displacement vs log time. g Slopes of the events in f, which represent the anomalous coefficient α, were averaged (n = 19). The average α of 1.49 ± 0.22 indicates that the events are not caused by a random diffusion process (α = 1). The motor protein dynein, known to power directed microtubule gliding, has a comparable α (1.65 ± 0.18, n = 11). Error bars show the standard deviation. Source data are provided as a Source Data file.