Abstract
In present investigation, the effort is done to enhance the grain quality in brewer’s rice cultivar Yamadanishiki with the plasma treatment of caryopsis (rice fruit) on ripening process. Seedlings transplanted from a paddy field into pots were grown in a greenhouse, and each caryopsis was treated with plasma on 1, 5, 10 and 15 days after flowering (DAF). The ratio of white-core grains to total number of grains was decreased in the grains treated on DAF1, same level on DAF5, and increased on DAF10 and 15, respectively, compared with control grains. Moreover, same treatment test was conducted with seedlings transplanted from a paddy field into pots and grown in growth chambers equipped with a sensing system to monitor environmental and growth conditions, referred to the climatic conditions of paddy fields. The ratio of white-core grains to total number of grains was decreased in the grains treated on DAF1, and increased on DAF5, 10 and 15, respectively. We demonstrated that plasma treatment of caryopsis affected the formation of white core, and that environmental conditions in the growth chamber were simulated to a paddy field. We would advocate the next-generation agriculture using ICT and plasma, “Smart Agriculture System”, for producing high-quality crops.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78620-y.
Keywords: Cold plasma application to agriculture, Grain quality enhancement, Brewer’s rice cultivar, Data sensing, Smart agriculture
Subject terms: Biological techniques, Biophysics, Biotechnology, Plant sciences
Introduction
Non-equilibrium atmospheric-pressure plasma (cold plasma) can generate a variety of reactive species, such as electrons, ions, and radicals under atmospheric pressure and near room temperature, and, therefore, it has the high reactivity. Consequently, this technology has been applied extensively in fields such as semiconductors, functional materials, environmental science, and biotechnology, fostering numerous technological advances. In particular, compared with the conventional thermal equilibrium plasma, since the rapid process by the reactive species can be conducted conveniently and at low-cost without vacuum equipment or chambers, cold plasma can expect to be applied in a variety of fields. In the area of semiconductor and functional device production, cold plasma has been used extensively in surface treatment methods, such as cleaning, etching, and modifications required for bonding dissimilar materials, bringing innovation to the industry1. In recent years, the development of plasma applications that can be performed under ambient temperature and pressure conditions has also progressed, and its application in the biotechnology field, notably in areas such as medicine and agriculture, has attracted considerable attention. In particular, it is expected to bring about a wide range of technological innovations spanning from pre-harvest to post-harvest, due to its varied effects on agricultural applications2–5, including applications related to plant breeding through the introduction of proteins6, improvement in germination7–10, promotion of plant growth, pathogen sterilization11–14, and the retention of food freshness15. Among these, the promotion of plant growth by plasma treatment has attracted considerable attention as a revolutionary technology in traditional agriculture, providing safe and secure food without the use of genetic modification or genome editing. For example, it has been reported that plasma treatment promotes the growth of plant species such as pea7, wheat8, Arabidopsis thaliana9, radish16–19, barley20, tomato12,21, carrot22, rye23, rice24, soybean25, and sunflower26,27. This growth-promoting effect in different plant species has been recognized as a universal fact, and considerable research and development is currently being conducted in this area worldwide. However, many of these studies focused on initial growth after germination in the laboratory or in the growth chamber, and few have examined the effect of plasma treatment on harvest. Use of growth chamber has many advantages because the environment can be controlled, the investigations can be continuously conducted, and the results can be reproduced. On the other hand, field tests are conducted in a natural environment and climate, so they can be conducted in accordance with an actual cultivation. However, many environmental factors, such as rain and wind, influence on the results, so it must be careful to determine whether the plasma is effective or not.
Our group has focused on each growth stage of rice (Oryza sativa L.), and has studied the effects of plasma treatment on brewer’s rice cultivation Yamadanishiki called the “king of sake rice”28. Rice is one of the world’s major cereal crops, and its cultivation process consists of multiple steps, spanning seed germination and seedling growth in a greenhouse, transplanting and growth in a paddy field, and finally harvest29. To demonstrate the effectiveness of plasma in agriculture, it is necessary to clarify the effect on each growth stage and to optimize plasma treatment conditions. In particular, the process following the transplantation of seedlings into paddy fields is accompanied by a dramatic shift from vegetative growth to reproductive growth, primarily due to the transition in climate from summer to autumn and other factors directly related to harvest29,30. In addition, climate warming has caused to shorten the period of rice cultivar31. Previously, it was unclear at which developmental stage optimal plasma treatment should be administered, and whether the effectiveness of plasma treatment would be manifested. Another factor requiring consideration is that there are two methods of plasma application in agriculture. One is the direct treatment method, in which seeds and seedlings are treated directly with plasma, and the other is the indirect method, in which plasma is used to treat water or a solution, which is then applied to seeds and seedlings. We previously treated rice plants in a paddy field by direct plasma treatment and plasma-activated solutions, and showed that both treatments not only improved growth promotion and yield, but also those grain quality32,33.
Seedlings of cooking rice cultivar (cv. Aichinokaori) that were planted in a test paddy field at Nagoya-University farm were treated with cold plasma in two ways32: direct plasma treatment of the shoot apical meristem (SAM) and indirect plasma treatment by immersion in a plasma-activated Ringer’s lactate solution (PAL). We previously discovered PAL in medical research, where we tested its effect on the selective induction of cancer cell death without harming normal cells34–36. We were the first to apply this solution, which has a revolutionary impact on human cells, for use in plants and to demonstrate its effectiveness. The findings of those studies showed that direct plasma treatment resulted in an increase in weight of plant whole body by 14.6% and yielded brown rice weight by 15.4%, as well as a significant decrease in the ratio of immature grains to the total number of grains by 2.1%. PAL treatment by immersion of rice seedling resulted in the improvement of traits related to the growth of the main stem and the decrease of weight of whole plant body. Nevertheless, the grain quality was improved due to significant decrease of the ratio of immature grains to total number of grains by 1.9%. In direct plasma treatment, the reactive oxygen and nitrogen species (RONS), such as O(3Pj) and NO radicals, produced from the plasma source effectively stimulate the SAM. Our previous studies showed that the density of O(3Pj) and NO radicals were high37, and that the contribution of RONS produced from the plasma on the inactivation of P. digitatum spores13 and the activation of budding yeast cells38. In the case of direct plasma treatment of SAM, these radicals are considered to activate cytokinin biosynthesis, and result in the promotion of cell division in the meristem. In fact, we preliminary showed that the direct plasma treatment of rice seeds after absorbing water with plasma promoted the elongation of coleoptile after germination with the up-regulation of the synthesis of several plant hormones, such as auxin and cytokinin, in the embryo of seeds (unpublished data). On the other hand, we have investigated the PAL solution; several chemical compounds are produced during the preparation of PAL solution with plasma including pyruvate, formate, acetate, glyoxylate, and 2,3-dimethyl tartrate and 2-(methylamino) ethanol36,39,40. These chemicals or other substances in PAL can affect the growth of rice plants as well as that of cancer cells, by functioning as some kind of plant hormones, such as auxin, cytokinin, and abscisic acid, and these may affect cellular functions in the roots and/or SAM41,42. Thus, both direct plasma treatment of seedlings and the application of PAL can affect the growth, yield, and grain quality due to different efficacies.
In addition to a cooking rice cultivar, the effects of cold plasma by direct plasma treatment of SAM and immersion of seedlings in PAL solution in a test paddy field at Nagoya-University farm were tested using a representative brewer’s rice cultivar, Yamadanishiki. Direct plasma treatment resulted in the increase of brown rice weight by 8.0%, the significant reduction of the ratio of immature grains to total number of grains by 6.1%, and the increase of the increase of white-core grains to total number of grains by 7.2%, compared with those in control. In PAL treatment, the ratio of immature grains to total number of grains was significantly decrease by 4.2%, and the ratio of white-core grains to total number of grains was increase by 6.3%, compared with those in the seedlings treated with distilled water. Therefore, cold plasma treatment in a paddy field also affected yield and quality of grain on the brewer’s rice cultivar, as well as cooking rice cultivar. As described below, white-core grains are preferred for producing Japanese rice wine (sake) and are typically found in the grains from brewer’s rice cultivars. These results suggest that direct plasma treatment exerts a specific stress/stimulus to the site where new cells and tissues develop, such growth points are two major quantitative trait locus (QTLs) essential for regulating 100-grain weight (GWt) and grain width (GWh) were harboured in the same regions on chromosomes 5 and 1043, and that PAL treatment has a plant-hormone-like effect after absorption from the roots. Thus, both mechanisms promote growth and improve the quantity and quality of the harvest. The expression of white cores varies significantly with the growth environment and climatic conditions44,45.
Among these results, the structural regulation of white core is a crucial factor in sake production. White core refers to a structure characterized by incomplete starch crystallization, which manifests as voids in the center of the endosperm46,47. Unlike cooking rice cultivars, brewer’s rice cultivars have a tendency to develop a white core in the endosperm48, and these rice grains are a preferred raw material for producing sake. In sake production, steaming of rice grains is followed by saccharification thorough the action of koji mold (Aspergillus sp.) and fermentation by yeast (Saccharomyces cerevisiae). In the initial stage of this process, the white-core grain structure is considered important for ensuring that the process proceeds smoothly and evenly from both the outside and inside of the rice grain. Consequently, through direct plasma treatment and indirect PAL treatment applied after the transplanting stages of seedlings, we have successfully improved yields and grain quality in the brewer’s rice cultivar based on the results as described above.
In addition, one of the most important events in plant growth is development of seeds, which encompasses the process of flowering, pollination, and ripening. In rice plant cultivation, the caryopsis undergoes ripening for 40 days after the formation of primary endosperm nucleus by fertilization, to become mature grain49. Throughout this period, a variety of factors, such as plant growth and weather conditions, can significantly influence the degree of grain maturation. To date, there have been no studies investigating the treatment of the flower part after heading with cold plasma. Thus, in this study, at a first step, we treated each caryopsis with plasma at specified days after flowering (DAFs) in a greenhouse adjacent to the experimental field, and evaluated the weight and quality of the grains after harvest. In the second test, the same experiments were conducted in a growth chamber equipped with a high-intensity LED panel capable of supporting rice plant growth through to harvest, which is termed “Smart Agriculture System”. The high-intensity LED panel using in present study has stable and high illuminance even for long cultivation period, compared with conventional metal halide lamps, and, thus, is essential to accurately investigate the influences of environmental conditions, such as temperature and daytime, on the plant growth. Within this chamber, environmental conditions such as temperature, humidity, and photoperiod were controlled to simulate climatic conditions in the field. In addition, an array of sensing systems and a camera were used to monitor the plant growth and environmental conditions, and to aggregate this information as cultivation data; refer to the developed system as an “ICT dynamic image system”. Within the context of climate change and advancing sustainable development goals (SDGs) in agriculture, it is essential to establish control systems for monitoring the growth environment as well as the software required to manage these systems. Recently, big data in various aspects of agriculture, such as plant breeding50, and cultivation51, the prediction of optimization using the artificial intelligence (AI) has much attention. Similarly, with the continuous investigations for optimizing the plasma treatment methods to produce high-quality crop in various cultivation environments, and based on those given data, the novel plasma technology in agriculture can be innovated such as the creation of cultivation recipes. Utilizing this system in the present study, we introduce the concept of “Smart Agriculture System” and validate its efficacy.
Results
Enhancement of the grain quality in brewer’s rice cultivar through direct plasma treatment of caryopsis
In Fig. 1 upper part, the cultivation schedule for the brewer’s rice cultivar (Oryza sativa L., cv. Yamadanishiki) and plasma treatment are shown. Briefly, young seedlings of Yamadanishiki were transplanted in a paddy field at the Togo Field for Science and Education, Nagoya University, Japan, on June 20, 2017. Then, on August 10, 2017, 20 seedlings of them were individually transplanted to 1/5000a Wagner’s pots (16 cm in a diameter, 19 cm in a height) on August 10, 2017, and grown in a greenhouse adjacent to the paddy field (Fig. 2a). On Aug 19, 2017, granular fertilizer was once added around stems on the top of soil in each pot. After start of heading on August 30, 2017, the flowering glumes were marked with aqueous pens (Fig. 2b). The colors and marks were changed in order to identify the dates from August 31 to September 12, 2017, as shown in Supplementary Fig. S1. Among them, each caryopsis was correctly treated with plasma by pen-type He plasma jet52,53 at different flowering days after flowering (DAFs) of 1, 5, 10, or 15 days from respective flowering dates. (Fig. 3). After ripening period of approximately 40 days, the seeds were individually harvested from October 18 to 25, 2017. We collected 518 control seeds, and 50, 59, 53, and 87 seeds with plasma treatment at DAF 1, 5, 10, and 15, respectively.
Fig. 1.
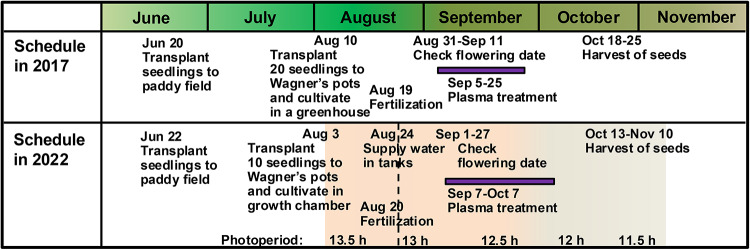
Cultivation and plasma treatment schedule for Yamadanishiki seedlings. Seedlings from a paddy field were transplanted into Wagner’s pot and then grown in greenhouse (Togo, 2017) and in a growth chamber referred to as a “Smart Agriculture System” (2022).
Fig. 2.
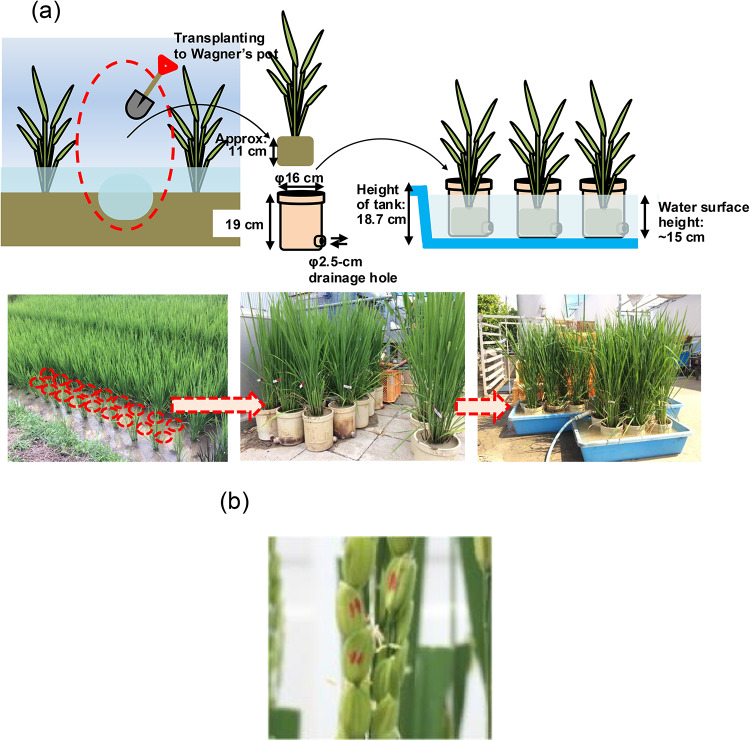
Preparation of samples cultivated in a paddy field. (a) Schematic diagrams (upper) and photographs (lower) of transplanting seedlings from a paddy field into Wagner’s pots. (b) Photograph of caryopses marked with an aqueous pen to identify the flowering date.
Fig. 3.
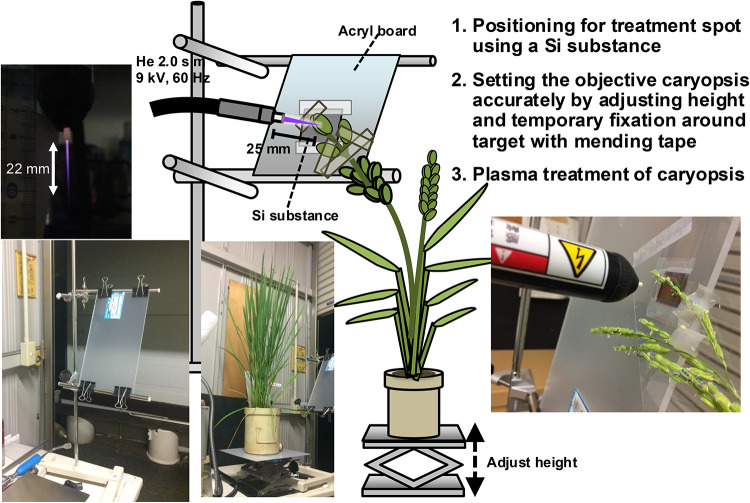
Experimental setup and plasma treatment of caryopsis using a pen-type He plasma jet.
Each seed was hulled by hand and brown rice was prepared. To evaluate the efficacy of plasma treatment for formation of brown rice quantitatively and qualitatively, weight and quality of the brown rice were investigated. Grains were classified into three categories based on their appearance normal, chalky, and immature grains based on the appearance (Fig. 4a). Although, in general, the chalky group included white-core, white-belly, white-back, and basal-white grains, white-back and basal-white grains were not detected in this study. The weight of each grain was measured and shown in Fig. 4b. In general, white-core grains are a characteristic of brewer’s rice cultivars, such as Yamadanishiki, as a high ratio of white-core grains is essential for brewing high-quality Japanese rice wine (sake). On the other hand, immature, and other chalky grains are not useful for sake brewing. The ratio of each category to the total number of grains was calculated for each treatment condition. Figure 4b shows the weight of a grain of brown rice. The average weight of 518 control grains and 50, 59, 53, and 87 grains with plasma treatment at DAF 1, 5, 10 and 15 were 24.5, 24.2, 24.0, 24.4 and 24.1 mg, respectively, and there were no significant differences in grain weight compared with control and plasma-treated grains under any plasma conditions by Student’s t test. Figure 4c shows the ratio of white-core grains to the total number of grains. For the control grain, it was 30.9%, while those for plasma-treated grains at DAF 1, 5, 10, and 15 were 21.3%, 28.8%, 46.0%, and 43.0%, respectively. Compared to the control grains, the ratios were lower in the grains treated at DAF 1 and 5 days and higher at DAF 10 and 15 (See also Supplementary Table S1).
Fig. 4.
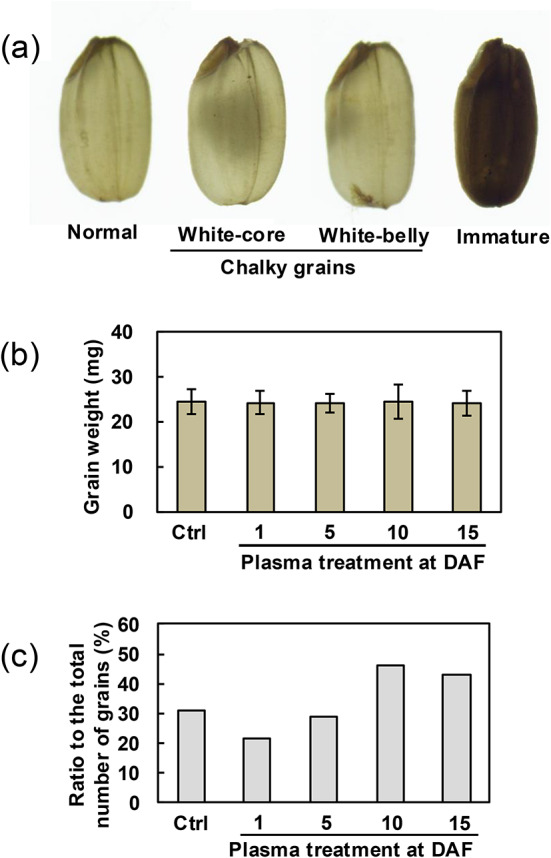
Variation in states of brown rice assessed for quantity and quality following plasma treatment of plants grown in a greenhouse. (a) Transmission microscope images of normal, chalky (white-core and white-belly), and immature grains; (b) Grain weight of brown rice; and (c) Ratio of white-core grain to the total number of grains in respective condition. For statistical analysis, Student’s t test was used to compare the weight between control and plasma-treated grains in each condition.
Variation in white core formation identified based on image analysis of transmission images
Based on the results in the previous section, the effects of plasma treatment of caryopsis at different DAFs on the formation of white core in the endosperm were analyzed using transmission images of brown rice. Transmission images of each grain were subjected to image analysis to assess the formation of white core. Immature grains were excluded for the analysis due to insufficient crystallization inside the whole grain, and normal and chalky grains were used for the analysis (See Fig. 4a). As shown in Fig. 5a, endosperm composed of fully crystallized starch transmits light, whereas white core is shaded because the starch is poorly crystallized and contains voids. The widths of these grains were almost same by averaging 3.27 mm and 3.23 mm (data not shown), respectively, and the line profiles were plotted by standardizing those equatorial planes to 500 pixels. As shown in Fig. 5b, a grayscale line profile between both edges in the equatorial plane from belly side to back side was created for each grain and the grayscale values were averaged for each treatment condition. The analysis of the control grains revealed that the formation of the white core was characterized by two peaks within the region extending from slightly inside the belly side to the depression of the palea. Compared with the results obtained for the control grains, the peaks in the treated grains at DAF 1 or 5 exhibited reductions in both the vertical and horizontal axes, suggesting the formation of void spaces in the white core occurred over a smaller area within the endosperm. On the other hand, the peaks in the grains treated at DAF 10 or 15 expanded on both the vertical and horizontal axes, suggesting a more extensive formation of the white core across a larger and wider area. These results were consistent with the classifications of grain appearance shown in Fig. 4b.
Fig. 5.
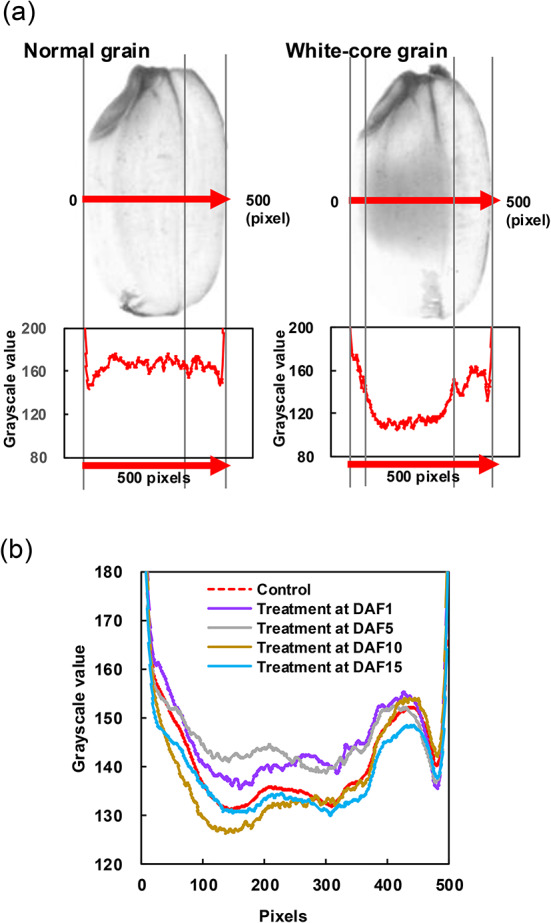
Variation of white core formation in the endosperm with plasma treatment of caryopsis at different DAFs by image analysis. (a) Line profile extracted from a grayscale transmission image of brown rice in the equatorial plane from the belly to the back side by normalizing the image to 500 pixels; e.g.) normal (left) and white-core (right) grains. (b) Line profiles for each treatment condition obtained by averaging the values from all mature brown rice.
Construction of “Smart Agriculture System” using on-farm data as a recipe
We conducted same investigations in a growth chamber, which we refer to here as a “Smart Plant”. In addition, we constructed the “Smart Agriculture System” (Fig. 6a), which was composed with a fixed-point camera, various sensors, and a workstation to collect and manage the data. Figure 1 lower part shows the cultivation schedule for the brewer’s rice cultivar (Oryza sativa L., cv. Yamadanishiki) and plasma treatment. Briefly, young seedlings of Yamadanishiki were transplanted in a paddy field at the Togo Field for Science and Education, Nagoya University, Japan, on June 22, 2022. On August 3, 2022, 10 seedlings of them were individually transplanted to 1/5000a Wagner’s pots. Five of 10 pots were transferred to two Smart Plants and grown. As shown in Table 1, referring to the climatic data in Togo Town, Aichi Prefecture, conditions such as temperature, humidity, and daylight were configured in five daily intervals from August 3 to November 10. As shown in Fig. 6b, water was poured onto each Wagner’s pot, which was covered with a rubber plug, up to the top using a water can from August 3 to 24, 2022. Then, the drainage holes were released on August 24, and water was supplied to the tank at a depth of approximately 15 cm to immerse the stems of plants. Values from the pH, water level, and water temperature sensors were set into the water in the tank, and started to record. On Aug 20, 2022, 3 g of granular fertilizer (16% nitrogen, 0% phosphorus, and 10% potassium) was once added around stems on the top of soil in each pot. After start of heading on August 31, 2022, the flowering glumes were marked with aqueous pens of different colors as described above from September 1 to 27, 2022. Then, each caryopsis was correctly treated with plasma by pen-type He plasma jet at different DAFs of 1, 5, 10, or 15 days from September 7 to October 7. After ripening period of approximately 40 days, the grains were individually harvested from October 13 to November 10, 2022. We collected 1879 control seeds, and 78, 59, 82, and 90 seeds with plasma treatment at DAF 1, 5, 10, and 15, respectively.
Fig. 6.
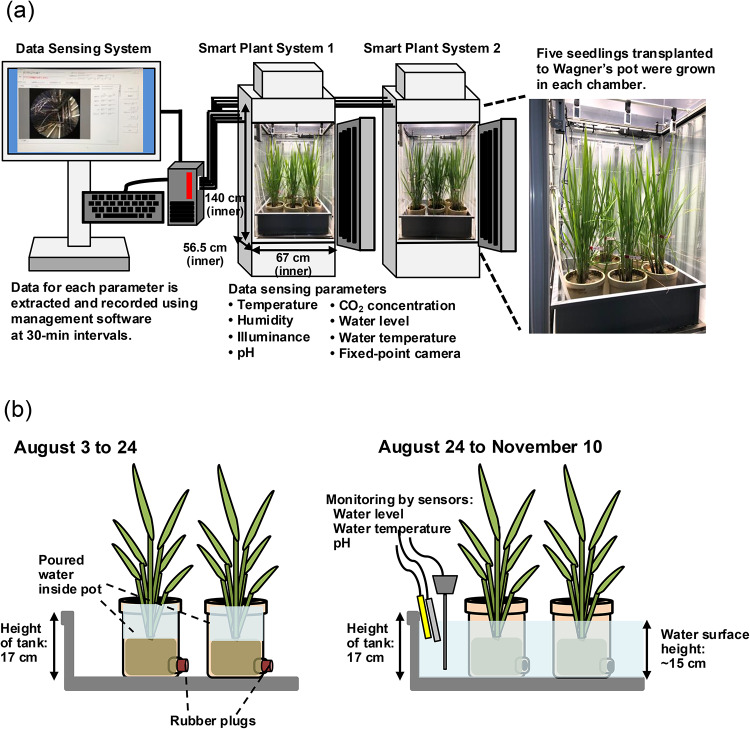
(a) Configuration of “Smart Agriculture System” used for plant growth and (b) schematic diagrams of water management in Smart Plant System from August 3 to 24 (left), and from August 24 to November 10 (right).
Table 1.
Setting of growth conditions in “Smart Agriculture System” from August 3 to November 10, 2022.
| Period | Parameters | Setting value | Daytime | ||||
|---|---|---|---|---|---|---|---|
|
Aug 3 to Aug 9 |
Time | 6:30 | 11:00 | 16:00 | 20:00 | 23:59 | 13 h 30 min |
| Temperature (°C) | 30 | 32 | 30 | 28 | 24 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | ++ | +++ | + | - | - | ||
|
Aug 9 to Aug 17 |
Time | 6:40 | 11:00 | 16:00 | 20:00 | 23:59 | 13 h 20 min |
| Temperature (°C) | 30 | 32 | 30 | 28 | 24 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | ++ | +++ | + | - | - | ||
|
Aug 17 to Aug 23 |
Time | 6:50 | 11:00 | 16:00 | 20:00 | 23:59 | 13 h 10 min |
| Temperature (°C) | 28 | 32 | 30 | 28 | 24 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | + | +++ | + | - | - | ||
|
Aug 23 to Aug 30 |
Time | 7:00 | 11:00 | 16:00 | 20:00 | 23:59 | 13 h 00 min |
| Temperature (°C) | 28 | 32 | 30 | 28 | 24 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | + | +++ | + | - | - | ||
|
Aug 30 to Sep 6 |
Time | 7:10 | 11:00 | 16:00 | 20:00 | 23:59 | 12 h 50 min |
| Temperature (°C) | 28 | 32 | 30 | 26 | 22 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | + | +++ | + | - | - | ||
|
Sep 6 to Sep 14 |
Time | 7:15 | 11:00 | 16:00 | 19:55 | 23:59 | 12 h 40 min |
| Temperature (°C) | 28 | 31 | 30 | 25 | 22 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | + | +++ | + | - | - | ||
|
Sep 14 to Sep 25 |
Time | 7:20 | 11:00 | 16:00 | 19:50 | 23:59 | 12 h 30 min |
| Temperature (°C) | 28 | 31 | 30 | 25 | 22 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | + | +++ | + | - | - | ||
|
Sep 25 to Oct 2 |
Time | 7:40 | 11:00 | 16:00 | 19:50 | 23:59 | 12 h 10 min |
| Temperature (°C) | 28 | 31 | 30 | 25 | 22 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | + | +++ | + | - | - | ||
|
Oct 2 to Oct 12 |
Time | 7:50 | 11:00 | 16:00 | 19:50 | 23:59 | 12 h 00 min |
| Temperature (°C) | 26 | 28 | 28 | 25 | 22 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | + | +++ | + | - | - | ||
|
Oct 12 to Oct 19 |
Time | 7:55 | 11:00 | 16:00 | 19:45 | 23:59 | 11 h 50 min |
| Temperature (°C) | 26 | 28 | 28 | 25 | 22 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | + | +++ | + | - | - | ||
|
Oct 19 to Oct 26 |
Time | 8:00 | 11:00 | 16:00 | 19:40 | 23:59 | 11 h 40 min |
| Temperature (°C) | 24 | 28 | 25 | 23 | 20 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | + | +++ | + | - | - | ||
|
Oct 26 to Nov 10 |
Time | 8:05 | 11:00 | 16:00 | 19:40 | 23:59 | 11 h 30 min |
| Temperature (°C) | 24 | 27 | 25 | 23 | 20 | ||
| Humidity (%) | 80 | 80 | 80 | 80 | 80 | ||
| Side LED lightning | + | +++ | + | - | - | ||
Figure 7 shows the cultivation environment data that were collected: (a) temperature, (b) humidity, (c) illumination, (d) CO2 concentration, (e) water level, (d) pH, and (g) water temperature. The minimum and maximum temperatures were obtained to decrease as cultivation duration progressed (Fig. 7a). Illumination from the high-intensity LED was set to 40,000 lx at 30 cm below the light source. As the rice seedlings grew, the sensor was shaded and values could not be obtained on some days (Fig. 7c). In this study, since CO2 levels were not regulated, its concentration naturally decreased to a minimum of approximately 150 ppm in August and September during the day and increased to approximately 500 ppm at night, differing from the ambient air concentration of approximately 400 ppm. This pattern, which showed a marked difference of approximately 350 ppm between day and night until the end of September and a smaller difference after October, is shown in Fig. 7d. In addition, a record of the water level following watering on August 24 is shown in Fig. 7e. The water supply was configured so that the bases of the seedlings’ stems in the pots due to the height of approximately 11 cm was remained continuously submerged with levels remaining in the range of 110–150 mm. The pH value for the water remained fairly constant (Fig. 7f). The water temperature also decreased as cultivation progressed (Fig. 7g). As a result of growing the seedlings in this environment, as shown in Fig. 1, growth was observed to progress in a similar manner to our previous report33 and experiments conducted in a greenhouse environment in 2017. In addition to those collecting data from sensors, the growth of seedlings was recorded by fixed-point camera inside each chamber (Supplementary Fig. S2). Major items regarding the phenology of rice cultivation, such as the initiation of heading and the date of harvest, were observed occurring on September 1 and October 13, respectively.
Fig. 7.
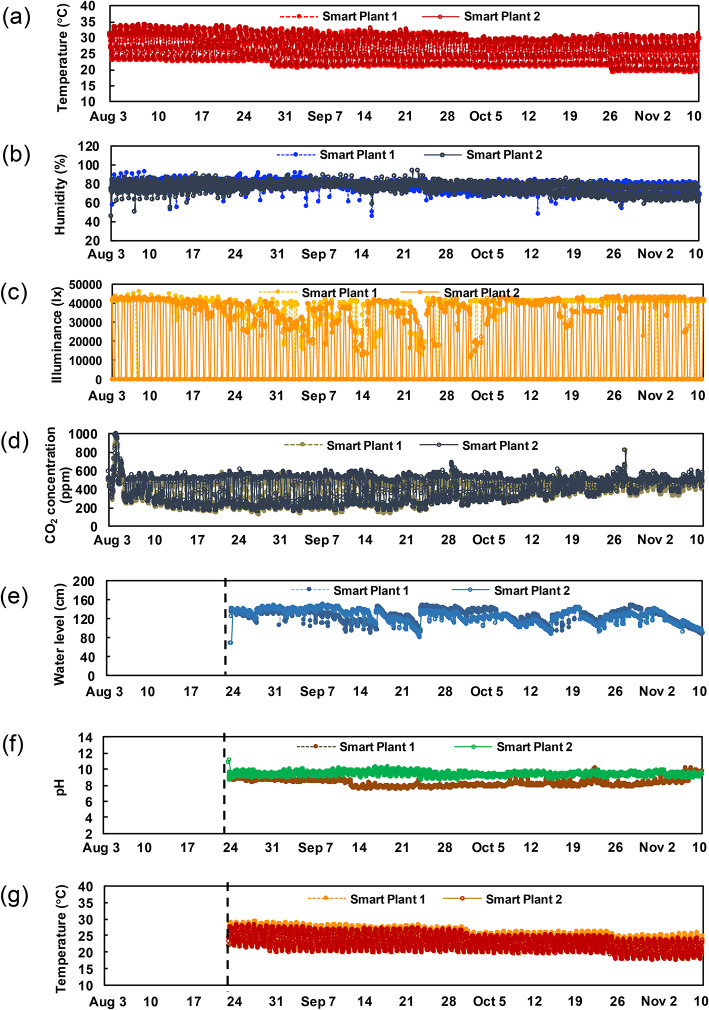
Sensing data captured by the ICT dynamic imaging system throughout the cultivation period in two plasma smart plants from August 3 to November 10; (a) Temperature, (b) humidity, (c) illuminance, (d) CO2 concentration, (e) water level, (f) pH, and (g) water temperature.
Enhancement of grain quality through post flowering direct plasma treatment and grown in the Smart Agriculture System
As described above, each grain of collected brown rice was weighed (Fig. 8a). The average weight of 1879 control grains and 78, 59, 82, and 90 grains with plasma treatment at DAF 1, 5, 10 and 15 were 21.6, 19.7, 18.8, and 21.2 mg, respectively, and there were no significant differences compared between control and plasma-treated grains under any plasma conditions with Student’s t test. Figure 8b shows the ratio of white-core grains to the total number of grains. In the control group, the ratio of white-core grains was 4.3%, whereas the ratios for the plasma-treated grains at DAF 1, 5, 10 and 15 were 2.6%, 27.1%, 22.0%, and 32.2%, respectively. The results indicated that the expression of white-core grains tended to decrease when treatment was applied 1 day after flowering, and increased with treatment administered after DAF 5.
Fig. 8.
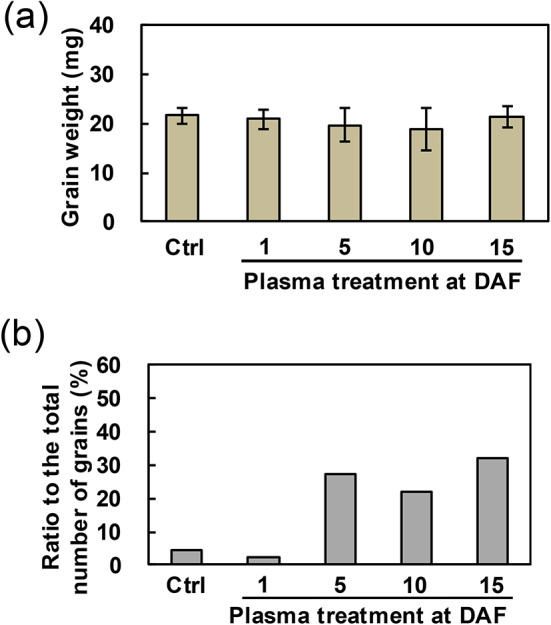
Changes in quantity and quality of brown rice due to plasma treatment on plants cultivated in Smart Agriculture System. (a) Grain weight of brown rice; and (b) Ratio of white-core grain to the total number of grains in respective condition. For statistical analysis, Student’s t test was used to compare the weight between control and plasma-treated grains in each condition.
Discussion
In this study, from twice tests in 2017 and 2022, we showed that the first investigations of direct plasma treatment of caryopses of rice seedlings after flowering improved the grain quality of a brewer’s rice cultivar. In both tests, to treat each caryopsis precisely with plasma, seedlings were transplanted from a paddy field to Wagner’s pots before heading. And then, they were grown at first test in a greenhouse in 2017 and at second test in Smart Agriculture System in 2022, respectively, until harvest. Given the close proximity between the experimental paddy field and the greenhouse, the environmental conditions in both stages of the study (i.e., experiments in the field and experiments in the greenhouse) were similar. In the control grains, the ratio of white-core grains to the total number of grains was 30.9%, which was similar to that observed in our previous study (32.5%, Ref.33). Thus, it was confirmed that the growth and the ripening of the grains from the transplanted seedlings in this study proceeded normally (Fig. 4b). Endosperm formation after pollination proceeds as follows30; after flowering, the embryo and endosperm are fertilized separately by self-pollination, and the nucleus in the endosperm divides without forming a cell membrane. The division progresses upward along the inner wall of the embryo sac until DAF 3 and towards the interior of the embryo sac until DAF 10, when the interior of the embryo sac is comprised of cells. The endosperm cells enter a phase of hypertrophic growth, in which photosynthetic products are translocated into the cells and ripening proceeds. After approximately another 30 days, a mature brown rice grain is formed. In this study, we focused on the process of endosperm formation and investigated the effects of plasma treatment of the caryopsis and the development of brown rice. Figures 5b and 6b showed that plasma treatment at DAF 1 decreased the ratio of the white-core grains to the total number of grains, while treatment at DAF 10 increased the ratio of white-core grains. These results imply that plasma treatment affected the endosperm formation and ripening phases through distinct mechanisms. During the endosperm formation stage, cell division commences following the multinucleation of the endosperm cells54. The equilibrium between the starch biosynthetic enzymes, BEIIE, and the hydrolysis enzyme α-amylase plays a critical role in starch crystallization throughout the ripening stage. Furthermore, Mn-type superoxide dismutase (MSD1), which is stimulated by stress factors such as high temperature, serves to regulates these enzymes55–59.
The He plasma jet used in this study was previously developed for medical applications, such as denaturing and aggregating proteins, and stopping bleeding60. It has also been reported to induce the proliferation of mouse neurons61. When plasma discharge is performed at a He gas flow rate of 2.0 slm, the discharge zone is approximately 22 mm. In this study, we set the treatment distance at 25 mm, ensuring that the plasma plume did not contact the caryopsis. In our previous studies, the density of oxygen radicals (O(3Pj)) measured by vacuum ultraviolet absorption spectroscopy (VUVAS) revealed that the densities of the generated radicals were on the order of 1013 cm−352,53. In addition to O(3Pj), O3 could be produced by reaction with atmospheric oxygen62. Furthermore, N(4So) and NO radicals were detected in the measurements of reactive oxygen and nitrogen species produced by atmospheric pressure Ar plasma at 60 Hz and 9 kV37, implying that these radicals would also be generated with this He plasma jet. It is suggested that, during the treatment of caryopses with plasma in rice plants at different DAFs, these reactive species affected gene expression and the activity of enzymes associated with cell division and starch synthesis/degradation during endosperm formation. Further molecular analysis is needed to elucidate which plasma-induced mechanisms affect the formation and ripening the endosperm.
Grayscale image analysis revealed more objective effects on the region of white core formation (transverse direction) and crystallization (vertical direction) (Fig. 5b). To analyze the crystallization and void structure in the white core more precisely, it will be necessary to observe them by electron microscopy.
Similar experiments were conducted using the growth chamber, Smart Agriculture System. In general, investigations using growth camber have conducted under the environmental conditions by optimizing temperature, humidity and daylength. On the other hand, there have been some difficulties on growing rice plants in the laboratory due to high light intensity requirements63–65. Thus, this is the first investigation to be conducted with the idea of reproducing the climate in a specific region. Figure 7 shows that most of the measured parameters were recorded accurately, although only the values in illuminance were not stably recorded due to the physical reasons by samples as described below. In the present experiments, CO2 concentrations were not regulated, and the diurnal variation was considerable by a maximum of approximately 350 ppm due to the influence of the plants’ own metabolic activities (e.g., photosynthesis in the daytime and respiration at night); consequently, it may have differed from levels in the field (constant at 400 ppm). Indeed, the weight of control grain harvested from plants cultivated in the Smart Agriculture System was slightly less than that of plants cultivated in the field (Figs. 4b and 8a), and the ratio of white-core grains to the total number of grains in control brown rice from the Smart Agriculture System was also lower than that obtained from plants grown in the field (Figs. 4c and 8b). The findings suggested that the environment in the Smart Plant had some influences on plant growth. Nevertheless, similar tendencies were observed in other characteristics, such as the dates of heading and variation in the expression of white core by plasma treatment at different DAFs. In this study, sensors of water level, water temperature, and pH were installed in the water outside pots in the tank. To monitor the plant growth in detail, it is important to measure the status of the soil. Thus, the results demonstrated that the growth environment in the field (Togo Town) was successfully reproduced by the Smart Agriculture System equipped with the ICT dynamic imaging system, although there should be still some rooms for improvement. In addition, in this study, less than 100 plasma-treated samples in any conditions among twice tests were be prepared due to technical difficulties for the setting up accurate plasma treatment of caryopsis. However, the variation of the ratio of white-core grain to the total number of grains had similar tendencies as mentioned above throughout twice tests. Therefore, those obtained results in this study are reliable. By utilizing this system, it would be possible to reproduce the climate of any regions in the world and to conduct cultivation trials under weather conditions that reflect those predicted for the future without any concerns reflected to cultivation seasons. Since the accumulation of cultivation data is an important component of developing ICT-based smart agriculture methodologies based on cold plasma, this system has the potential for making a significant contribution. As a future issue to obtain stable results, the improvements are needed to treat caryopses more efficiently, and, based on the prepared sufficient samples, to discuss statistically, i.e., by grouping some seedlings.
In this study, we showed that direct plasma treatment of caryopsis of Yamadanishiki, a brewer’s rice cultivar, at different DAFs can control both increase and decrease of the development of white-core grain, by changing the treatment condition depending on the DAF (Figs. 4c and 8b). Furthermore, considering the results of the reduction of white core formation from the perspective of chalky grains, the results suggested that it can also potentially be applied to the improvement of cooking rice cultivars. The current challenge of increase in temperature referred to colloquially as “global boiling”, the agricultural sector faces significant issues. For examples, high temperature stress during the ripening period can cause the sterility and inadequate ripening, which significantly reduces yield and quality55,56,66–68. In the near future, it is considered important to conduct similar investigations using cooking rice cultivars. In addition, it is necessary to analyze the effects on not only the grain shape but also the components in brown rice. There has been reported that exposure of rice seedlings on various growth stages to ozone caused the change the ratio of carbon to nitrogen in brown rice69.
Similar with direct plasma treatment, the treatment of caryopses with PAL solution should be also investigated in the future. In our previous studies32,33, the grain quality was improved with the immersion of rice seedlings in a paddy field. Grain ripening is also expected to be promoted by the function like a plant hormone. In addition, based on the results that plasma treatment of caryopsis promotes the grain ripening, it is necessary to investigate the effects of the treatment on the growth of plants and the duration of ripening period from a point of view of phonology. Thus, the data obtained in the continuous tests using Smart Agriculture System, such as environmental conditions, plasma treatment conditions, growth of plants, and effects on the crop can be applied to AI to create the cultivation recipes. For practical use of cold plasma in Smart Agriculture System, it is necessary to reduce the cost, such as reactive gas, and develop the plasma device and treatment method for more efficient and large-scale treatment. Then, the Smart Agriculture System will be an effective tool to collect various data when the environment in the chamber can be controlled to be closer to that in the field.
In summary, we focused on the process of endosperm formation and ripening during the cultivation of the brewer’s rice cultivar, Yamadanishiki. We subjected each caryopsis to direct plasma treatment using a pen-type He plasma jet at different DAFs and assessed the efficacy of this treatment on the formation of white core in the brown rice. In the experiments on rice seedlings grown in a greenhouse adjacent to the university paddy field in Togo Town, Aichi Prefecture, plasma treatment at DAF 1 and 5 was observed to decrease the ratio of white-core grains to the total number of grains on seedlings. Conversely, treatment at DAF 10 and 15 increased the ratio of white-core grains. These results were substantiated by grayscale analysis using transmission images of white-core grains. In addition, we developed an integrated system filled with sensors that could be controlled using management software. This Smart Plant ICT Dynamic Image System can be used to monitor and manage data on growth conditions and cultivation environments. On the basis of the system, we further emulated the weather conditions of Togo Town and conducted experiments in the Smart Agriculture System that accurately reflected field conditions. This approach successfully replicated the findings, demonstrating that plasma treatment of caryopsis at different DAFs influences the formation of white core. These results suggest that applying plasma treatment to the rice cultivation process after heading has the potential to enhance grain quality. Furthermore, the advancement of agriculture through ICT in the future will benefit significantly from the implementation of the Smart Plasma Agriculture. This approach, which integrates an ICT dynamic imaging system and a data-driven methodology that combines optimal climatic and plasma treatment conditions, has been demonstrated to be effective.
Methods
Growth of brewer’s rice cultivar in a greenhouse
Figure 1 shows the cultivation schedule for the brewer’s rice cultivar (Oryza sativa L., cv. Yamadanishiki) and plasma treatment. The seeds were germinated on May 20, 2017, and grown in a greenhouse. Individual seedlings were then transplanted by hand at intervals within rows of 15 cm (D) and a distance between rows of 30 cm (W) in a rice paddy field at the Togo Field for Science and Education, Nagoya University, Japan, on June 20, 2017. After growing the seedlings in a paddy field using conventional cultivation methods, 20 plants were transplanted to 1/5000a Wagner’s pots on August 10, 2017 (Fig. 2a). The Wagner’s pot is cylindrical in ϕ16 cm of diameter and 19 cm of height with a ϕ2.5-cm drainage hole horizontally at the lower part. Each seedling was dug up with a shovel to a depth of approximately 11 cm along with a soil, and put in a pot. Twenty pots of seedlings were placed in three tanks with internal dimensions of 85.0 cm × 54.8 cm × 18.7 cm (W × D × H) and internal bottom dimensions of 79.0 cm × 49.0 cm (W × D), and subsequent growth was carried out in a greenhouse adjacent to the paddy field under controlled watering conditions. Water level was kept at the depth of approximately 15 cm by pouring water manually to immerse the stem of plant in water. On Aug 19, 2017, 3 g of granular fertilizer (16% nitrogen, 0% phosphorus, and 10% potassium) was added around stems on the top of soil each pot. After the initiation of heading on August 30, flowering glumes were marked with aqueous pens of different colors to visually identify the dates of flowering on August 31, September 4, 7, 8, 9, and 11, 2017; a total of 767 caryopses were marked in this way by changing colors and marks (See also Supplementary Fig. S1). Among the samples, of the marked caryopses 50, 59, 53, and 87 were treated with plasma at DAF 1, 5, 10, or 15, respectively. The rest 518 caryopses were used as control samples. Then, these mature seeds were individually harvested from October 18 to 25, 2017.
Growth of the brewer’s rice cultivar in the Smart Agriculture System using the ICT dynamic imaging system
As shown in Fig. 1, after the Yamadanishiki seeds were sown on June 2, 2022, the seedlings were grown in a greenhouse and transplanted to the Togo test paddy field on June 22. On August 3, 10 plants were transplanted into 1/5000a Wagner’s pots. Five plants were placed in each of two tanks (57 cm × 46 cm × 17 cm; W × D × H) in two growth chambers (Nippon Medical & Chemical Instruments Co., Ltd., LPH-411P-SPS, rectangular inner dimensions 67 cm × 56.5 cm × 140 cm; W × D × H) of the “Smart Plant” system, with controlled watering. The Smart Plant system is programmable for temperature, humidity, and day length. During the daytime, a high-intensity LED panel with 600 W (Nippon Medical & Chemical Instruments Co., Ltd., PFQ-600DT) installed above the seedlings was set to produce 40,000 lx at 30 cm below the light source. Four fluorescent LEDs (18 W × 16) were installed on each of the four sides of each system so that the lightning could be set to reflect conditions at different times of the day. As shown in Table 1, referring to climatic data from Togo Town, Aichi Prefecture, conditions such as temperature, humidity, and daylight were configured in five daily intervals to the time of day. These settings were typically changed at weekly intervals from August 3 to November 10. We set up a fixed-point camera and various sensors in the Smart Plant system, and connected them to a workstation with a program called the “ICT dynamic image system”, which we designed to collect and manage these data (Fig. 6). Specifically, the recording instruments inside the chamber comprised a fixed-point camera (DU657MC; Toshiba Teli, Tokyo, Japan); a data logger (TR-75Ui; T&D Corp., Nagano, Japan) to record temperature, humidity, and illumination; a CO2 meter (GCH-2018; Sato, Nagano, Japan), a pH meter (PH-230SD; Sato), a water level meter (FL001; Keyence, Osaka, Japan), and a water thermometer (E52; Omron, Kyoto, Japan), all of which were connected to a workstation (CELSIUS W570; Fujitsu, Kawasaki, Japan) with a management program (Mitani Corp., Fukui, Japan). Data was collected at 30 min intervals.
Initially, the drainage hole of each Wagner’s pot was covered with a rubber plug until August 24, 2022, and water was supplied from the base of the plants daily to the top of the pot using a watering can (See also Fig. 6b). On Aug 20, 2022, 3 g of granular fertilizer (16% nitrogen, 0% phosphorus, and 10% potassium) around stems on the top of soil each pot was added. Then, on August 24, 2022, the rubber plugs were removed from the drainage hole of pots. Water was supplied to the tank to a depth of 15 cm to immerse the stems of the plants. Values from the pH, water level, and water temperature sensors were set into the water in the tank, and recorded from that date. After the initiation of heading on September 1, 2022, flowering glumes were marked with aqueous pens of different colors as described above from September 1 to 27, 2022. Then, a total of 2188 caryopses were marked. Among the samples, of the marked caryopses 78, 59, 82, and 90 were treated with plasma at DAF 1, 5, 10, or 15 respectively. The rest 1879 caryopses were used as control samples. Then, these mature seeds were individually harvested from October 13 to November 10, 2022.
Plasma treatment of caryopsis
Figure 3 shows schematic diagram of the experimental setup used for direct plasma treatment of each caryopsis of rice plants. A 60 Hz, 9 kV pen-type He plasma jet treatment system was used for plasma treatment52,53. Firstly, to determine the treatment spot, a Si substance (2 cm square) was attached to an acrylic board with mending tape, and treated with plasma at a distance 25 mm. The obtained mark was designated as the position of plasma treatment. The potted rice plant was placed on a jack pedestal and adjusted in height, and the objective caryopsis for the plasma treatment was temporarily fixed with mending tape at the position so that plasma treatment could be applied to the center of a single caryopsis. The target caryopsis was treated with plasma for 20 s at a gas flow rate of 2.0 slm. Then, the caryopsis treated with plasma was marked with aqueous pens of different colors to visually indicate the treatment conditions, as shown in Supplementary Fig. S1. Preliminary studies using rice seeds confirmed that the temperatures on the surface of seeds were below 40 °C and that their germination and other aspects of growth were not influenced (data not shown).
Evaluation of harvested brown rice
To prepare the brown rice, the collected samples were air-dried for two weeks before being hulled manually and weighing each grain. To further evaluate the quality of the brown rice, rice grains was classified based on their appearance: normal, chalky (white-core, white-belly, white-back, and basal-white), immature, and broken grains. Each grain was visually sorted as below;
Normal grain: Milky and transparent grains.
White-core grain: Milky and transparent grains with a chalk in the center part.
White-belly grain: Milky and transparent grains with a chalk in the edge of embryo side.
White-back grain: Milky and transparent grains with a chalk in the opposite edge of embryo side.
Basal-white grain: Milky and transparent grains with a chalk around embryo.
Immature grain: brown or green appearance with skinny shape.
Broken grain: Grains broken inside caryopses.
The ratio of each type relative to the total number of grains in each treatment condition was then calculated.
For image analysis of the white core, transmission images of each brown rice grain were taken using a stereomicroscope (SZX16; Olympus, Tokyo, Japan), and those images were processed using a custom-made code based on the Igor Pro (version 8) software (WaveMatrics; Portland, USA). The images were firstly converted from red, green, and blue (RGB) colors into grayscale, subsequently extracting a line profile across the equatorial plane from the edge at the side with embryo (termed “belly side”) to the edge at the opposite side (termed “back side”). This profile was derived based on the grayscale values, which range from 0 to 255, with 0 representing pure black and 255 signifying pure white. For this analysis, normal and chalky grains were used, because those transmission images were taken due to sufficient starch crystallization in endosperm. Since the width of those grains was almost same, the length of the equatorial plane between both edges was standardized to 500 pixels, and then a line profile was drawn. Value of all measurement were averaged and plotted for each treatment condition.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported in part by Fujitsu Client Computing Limited, Enshu Co., Limited, and JSPS KAKENHI (Grant No. JP19H05462). Shinya Mizuno, Prof. Kazuyuki Doi, Prof. Shunsaku Nishiuchi, Dr. Yoichi Morinaka, Masaaki Kuge, and many staffs at the Togo Field for Science and Education of Nagoya University are thanked for their technical support with the cultivation of rice plants. Prof. Kenji Ishikawa, Prof. Hiroki Kondo, and Prof. Takayoshi Tsutsumi from Center for Low-temperature Plasma Sciences, Nagoya University, are thanked for plasma experimental configuration. Kuniaki Saito from Fujitsu Client Computing Limited, Akitoshi Itoh from the Food Research Centre, Aichi Centre for Industry and Science Technology, and Susumu Nikawa provided valuable advice regarding the direction of our research.
Author contributions
H. H. performed the experiments (plasma treatment, and individual investigations), and wrote the original draft. H. K. managed the plants in the paddy field, and reviewed the manuscript from the point of view of botanists. H. M. and A. A. were involved in various aspects of the investigation after harvesting the plants. E. K., G. Y., S. T., and Y. H. designed the experimental setup for the smart agriculture. H. T. and S.-N. H. reviewed this paper from the point of view of plasma engineers. S. M., H. S., and M. M. were proposed the experimental methods and the analysis and reviewed this paper from the point of view of botanists. M. M. was the project director for the agricultural applications of plasma and reviewed this manuscript from the point of a hospital professor. M. H. was a project manager, and reviewed and edited this paper.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hori, M. Radical–controlled plasma processes. Rev. Mod. Plasma Phys.6, 1–117. 10.1007/s41614-022-00084-2 (2022). [Google Scholar]
- 2.Ito, M., Oh, J. S., Ohta, T., Shiratani, M. & Hori, M. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Process. Polym.15, 1700073. 10.1002/ppap.201700073 (2018). [Google Scholar]
- 3.Adamovich, I. et al. The 2017 plasma Roadmap: low temperature plasma science and technology. J. Phys. D: Appl. Phys.50, 323001. 10.1088/1361-6463/aa76f5 (2017). [Google Scholar]
- 4.Park, D. P. et al. Reactive nitrogen species produced in water by non-equilibrium plasma increase plant growth rate and nutritional yield. Curr. Appl. Phys.13, S19–S29. 10.1016/j.cap.2012.12.019 (2013). [Google Scholar]
- 5.Puač, N., Gherardi, M. & Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym.15, 1700174. 10.1002/ppap.201700174 (2018). [Google Scholar]
- 6.Yanagawa, Y. et al. Direct protein introduction into plant cells using a multi-gas plasma jet. Pros One12, 0171942. 10.1371/journal.pone.0171942 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stolarik, T. et al. Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in Pea (Pisum sativum L.). Plasma Chem. Plasma Proc.35, 659–676. 10.1007/s11090-015-9627-8 (2015). [Google Scholar]
- 8.Jiafeng, J. et al. Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci. Technol.16, 54. 10.1088/1009-0630/16/1/12 (2014). [Google Scholar]
- 9.Koga, K. et al. Simple method of improving harvest by nonthermal air plasma irradiation of seeds of Arabidopsis thaliana (L). Appl. Phys. Exp.9, 016201. 10.7567/APEX.9.016201 (2016). [Google Scholar]
- 10.de Groot, G. J. J., Hundt, A., Murphy, A. B., Bange, M. P. & Mai-Prochnow, A. Cold plasma treatment for cotton seed germination improvement. Sci. Rep.8, 14372. 10.1038/s41598-018-32692-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, J., Sakai, N., Watanabe, M., Hotta, E. & Wachi, M. Study on plasma agent effect of a direct-current atmospheric pressure oxygen-plasma jet on inactivation of E. coli using bacterial mutants. IEEE Trans. Plasma Sci.41, 935–941. 10.1109/TPS.2013.2248395 (2013). [Google Scholar]
- 12.Shimada, K. et al. Humidification effect of air plasma effluent gas on suppressing conidium germination of a plant pathogenic fungus in the liquid phase. Plasma Process. Polym.17, 1900004. 10.1002/ppap.201900004 (2020). [Google Scholar]
- 13.Iseki, S. et al. Rapid inactivation of Penicillium digitatum spores using high-density nonequilibrium atmospheric pressure plasma. Appl. Phys. Lett.96, 153704. 10.1063/1.3399265 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashizume, H. et al. Inactivation effects of neutral reactive-oxygen species on Penicillium digitatum spores using non-equilibrium atmospheric-pressure oxygen radical source. Jpn. J. Appl. Phys.52, 056202. 10.1063/1.4824892 (2013). [Google Scholar]
- 15.Pankaj, S. K., Wan, Z. & Keener, K. M. Effects of cold plasma on food quality: A review. Rev. Foods7, 4. 10.3390/foods7010004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitazaki, S., Koga, K., Shiratani, M. & Hayashi, N. Growth enhancement of radish sprouts induced by low pressure O2 radio frequency discharge plasma irradiation. Jpn. J. Appl. Phys.51, 01AE01. 10.1143/JJAP.51.01AE01 (2012). [Google Scholar]
- 17.Kitazaki, S., Sarinont, T., Koga, K., Hayashi, N. & Shiratani, M. Plasma induced long-term growth enhancement of Raphanus sativus L. using combinatorial atmospheric air dielectric barrier discharge plasmas. Curr. Appl. Phys.14, S149–S153. 10.1016/j.cap.2013.11.056 (2014). [Google Scholar]
- 18.Hayashi, N., Ono, R., Shiratani, M. & Yonesu, A. Antioxidative activity and growth regulation of Brassicaceae induced by oxygen radical irradiation. Jpn. J. Appl. Phys.54, 06GD01. 10.7567/JJAP.54.06GD01 (2015). [Google Scholar]
- 19.Sarinont, T. et al. Effects of plasma irradiation using various feeding gases on growth of Raphanus sativus L. Arch. Biochem. Biophys.605, 129–140. 10.1016/j.abb.2016.03.024 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Park, Y. et al. The biological effects of surface dielectric barrier discharge on seed germination and plant growth with barley. Plasma Process. Polym.15, 1600056. 10.1002/ppap.201600056 (2016). [Google Scholar]
- 21.Adhikari, B., Adhikari, M., Ghimire, B., Park, G. & Choi, E. H. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci. Rep.9, 16080. 10.1038/s41598-019-52646-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takaki, K. et al. Improvements in plant growth rate using underwater discharge. J. Phys. : Conf. Ser.418, 012140. 10.1088/1742-6596/418/1/012140 (2013). [Google Scholar]
- 23.Lazukin, A. V., Serdyukov, Y. A., Moralev, I. A., Selivonin, I. V. & Krivov, S. A. Effect of the surface dielectric barrier discharge plasmas on winter rye seeds germination. J. Phys. : Conf. Ser.1147, 012124. 10.1088/1742-6596/1147/1/012124 (2019). [Google Scholar]
- 24.Khamsen, N. et al. Rice (Oryza sativa L.) seed sterilization and germination enhancement via atmospheric hybrid nonthermal discharge plasma. ACS Appl. Mater. Interfaces8, 19268–19275. 10.1021/acsami.6b04555 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Ling, L. et al. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep.4, 5859. 10.1038/srep05859 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mildažienė, V. et al. Treatment of common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep.9, 6437. 10.1038/s41598-019-42893-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zukiene, R. et al. Dielectric barrier discharge plasma treatment-induced changes in sunflower seed germination, phytohormone balance, and seedling growth. Appl. Phys. Exp.12, 126003. 10.7567/1882-0786/ab5491 (2019). [Google Scholar]
- 28.Anonymous. What is Yamadanishiki? The Genealogy of the. King of Sake Rice. Sake Street 11 (22) (2023). https://sakestreet.com/en/media/what-is-yamadanishiki (2024).
- 29.Itoh, J. et al. Rice plant development: From zygote to spikelet. Plant. Cell. Physiol.46, 23–47. 10.1093/pcp/pci501 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Hoshikawa, K. The Growing Rice Plant 260–261 (Nobunkyo, 1989).
- 31.Zhang, T., Huang, Y. & Yang, X. Climate warming over the past three decades has shortened rice growth duration in China and cultivar shifts have further accelerated the process for late rice. Global Change Biol.19, 563–570. 10.1111/gcb.12057 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Hashizume, H. et al. Improvement of yield and grain quality by periodic cold plasma treatment with rice plants in a paddy field. Plasma Process. Polym.18, 2000181. 10.1002/ppap.202000181 (2021). [Google Scholar]
- 33.Hashizume, H. et al. Efficacy of periodic cold plasma treatment in a paddy to produce white-core grains in brewer’s rice cultivar Yamadanishiki. Free Rad. Res.57, 161–173. 10.1080/10715762.2023.2215914 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Tanaka, H. et al. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep.6, 36282. 10.1038/srep36282 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka, H. et al. Oxidative stress-dependent and -independent death of glioblastoma cells induced by nonthermal plasma-exposed solutions. Sci. Rep.9, 13657. 10.1038/s41598-019-50136-w (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka, H. et al. Low temperature plasma irradiation products of sodium lactate solution that induce cell death on U251SP glioblastoma cells were identified. Sci. Rep.11, 18488. 10.1038/s41598-021-98020-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeda, K., Ishikawa, K., Tanaka, H., Sekine, M. & Hori, M. Spatial distributions of O, N, NO, OH and vacuum ultraviolet light along gas flow direction in an AC-excited atmospheric pressure Ar plasma jet generated in open air. J. Phys. D: Appl. Phys.50, 195202. 10.1088/1361-6463/aa6555 (2017). [Google Scholar]
- 38.Hashizume, H., Ohta, T., Hori, M. & Ito, M. Growth control of Saccharomyces cerevisiae through dose of oxygen atoms. Appl. Phys. Lett.107, 093701. 10.1063/1.4929952 (2015). [Google Scholar]
- 39.Ito, D. et al. Cytotoxicity of plasma-irradiated lactate solution produced under atmospheric airtight conditions and generation of the methyl amino group. Appl. Phys. Express15, 056001. 10.35848/1882-0786/ac6360 (2022). [Google Scholar]
- 40.Miron, C. et al. Cancer-specific cytotoxicity of Ringer’s acetate solution irradiated by cold atmospheric pressure plasma. Free Rad. Res.57, 91–104. 10.1080/10715762.2023.2201390 (2023). [DOI] [PubMed] [Google Scholar]
- 41.Mai, C. D. et al. Genes controlling root development in rice. Rice7, 30 (2014). http://www.thericejournal.com/content/7/1/30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao, G. et al. OsERF2 controls rice root growth and hormone responses through tuning expression of key genes involved in hormone signaling and sucrose metabolism. Plant. Mol. Biol.90, 293–302. 10.1007/s11103-015-0416-9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okada, S. et al. Genetic dissection of grain traits in Yamadanishiki, an excellent sake-brewing rice cultivar. Theor. Appl. Genet.130, 2567–2585. 10.1007/s00122-017-2977-2 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Ishii, K., Oba, K., Maruyama, A. & Katano, M. Effect of high temperature at grain filling period in TGC on grain texture of brewers’ rice Yarnada – Nishiki. Rep. Kyushu Br. Crop Sci. Soc. Japan74, 24–26 (2008). [In Japanese]. [Google Scholar]
- 45.Ikegami, M. et al. The relationships between temperature conditions and brown rice quality of a brewer’s rice cultivar Yamadanishiki in Hyogo prefecture. Jpn. J. Crop Sci.84, 295–302 (2015). [In Japanese]. [Google Scholar]
- 46.Horigane, A. K., Suzuki, K. & Yoshida, M. Moisture distribution in rice grains used for sake brewing analyzed by magnetic resonance imaging. J. Cereal Sci.60, 193–201. 10.1016/j.jcs.2014.02.011 (2014). [Google Scholar]
- 47.Yanagiuchi, T. et al. Influence of grain type on suitability of rice for sake brewing. Seibutsu-Kogaku Kaishi75, 169–176 (1997). [In Japanese]. [Google Scholar]
- 48.Ikegami, M., Yoshida, S., Nakamura, C. & Kamijima, O. Heritability estimates of white-core expression in a sake-brewing rice (Oryza sativa L.) cultivar Yamadanishiki based on F2 variance and selection response in the F2 generation. Breed. Res.5, 9–15 (2003). [In Japanese]. [Google Scholar]
- 49.Osada, A., Ishizaki, Y. & Suzuki, S. Difference in the number of days for ripening of grains between Japonica and Indica rice varieties. Jpn. J. Trop. Agr.27, 59–66 (1983). [Google Scholar]
- 50.Farooq, M. A. et al. Artificial intelligence in plant breeding. Trends Genet.40, 891-908. 10.1016/j.tig.2024.07.001 (2024). [DOI] [PubMed] [Google Scholar]
- 51.Jing Qiu, J., Lu, X., Wang, X., Chen, C. & Chen, Y. Q. Research on image recognition of tomato leaf diseases based on improved AlexNet mode. Heliyon10, e33555. 10.1016/j.heliyon.2024.e33555 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeda, K. et al. Behavior of absolute densities of atomic oxygen in the gas phase near an object surface in an AC-excited atmospheric pressure He plasma jet. Appl. Phys. Express10, 036201. 10.7567/APEX.10.036201 (2017). [Google Scholar]
- 53.Takeda, K. et al. Systematic diagnostics of the electrical, optical, and physicochemical characteristics of low-temperature atmospheric-pressure Helium plasma sources. J. Phys. D: Appl. Phys.52, 165202. 10.1088/1361-6463/aaff44 (2019). [Google Scholar]
- 54.Ohnishi, T. & Kinoshita, T. Early development of rice endosperm. Plant Morphol.22, 15–22 (2010). [In Japanese]. [Google Scholar]
- 55.Tanaka, N. et al. The structure of starch can be manipulated by changing the expression levels of starch branching enzyme IIb in rice endosperm. Plant Biotech. J.2, 507–516. 10.1111/j.1467-7652.2004.00097.x (2004). [DOI] [PubMed] [Google Scholar]
- 56.Ohdan, T., Sawada, T. & Nakamura, Y. Effects of temperature on starch branching enzyme properties of rice. J. Appl. Glycosci.58, 19–26. 10.5458/jag.jag.JAG-2010_014 (2011). [Google Scholar]
- 57.Asatsuma, S., Sawada, C., Kitajima, A., Asakura, T. & Mitsui, T. α-Amylase affects starch accumulation in rice grains. J. Appl. Glycosci.53, 187–192. 10.5458/jag.53.187 (2006). [Google Scholar]
- 58.Hakata, M. et al. Suppression of α-amylase genes improves quality of rice grain ripened under high temperature. Plant Biotechnol. J.10, 1110–1117. 10.1111/j.1467-7652.2012.00741.x (2012). [DOI] [PubMed] [Google Scholar]
- 59.Shiraya, T. et al. Golgi/plastid-type manganese superoxide dismutase involved in heat-stress tolerance during grain filling of rice. Plant Biotechnol. J.13, 1251–1263. 10.1111/pbi.12314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyamoto, K. et al. Red blood cell coagulation induced by low-temperature plasma treatment. Arch. Biochem. Biophys.605, 95–101. 10.1016/j.abb.2016.03.023 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Yamato, M. et al. Brain cell proliferation in adult rats after irradiation with nonequilibrium atmospheric pressure plasma. Appl. Phys. Express14, 067002. 10.35848/1882-0786/ac03c1 (2021). [Google Scholar]
- 62.Iwasaki, M. et al. Nonequilibrium atmospheric pressure plasma with ultrahigh electron density and high performance for glass surface cleaning. Appl. Phys. Lett.92, 081503. 10.1063/1.2885084 (2008). [Google Scholar]
- 63.Numajiri, Y. et al. iPOTs: internet of things-based pot system controlling optional treatment of soil water condition for plant phenotyping under drought stress. Plant. J.107, 1569–1580. 10.1111/tpj.15400 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Soma, F., Kitomi, Y., Kawakatsu, T. & Uga, Y. Life-cycle multiomics of rice shoots reveals growth stage–specific effects of drought stress and time–lag drought responses. Plant Cell Physiol.65, 156–168. 10.1093/pcp/pcad135 (2024). [DOI] [PubMed] [Google Scholar]
- 65.Fu, X., Zhong, L., Wang, H., He, H. & Chen, X. Elucidation of the mechanism of rapid growth recovery in rice seedlings after exposure to low-temperature low-light stress: analysis of rice root transcriptome, metabolome, and physiology. Int. J. Mol. Sci.24, 17359. 10.3390/ijms242417359 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishimaru, T. et al. Formation of grain chalkiness and changes in water distribution in developing rice caryopses grown under high-temperature stress. J. Cereal Sci.50, 166–174. 10.1016/j.jcs.2009.04.011 (2009). [Google Scholar]
- 67.Tabata, M. et al. Mapping of quantitative trait loci for the occurrence of white-back kernels associated with high temperatures during the ripening period of rice (Oryza sativa L). Breed. Sci.57, 47–52. 10.1270/jsbbs.57.47 (2007). [Google Scholar]
- 68.Suriyasak, C. et al. Reactive oxygen species induced by heat stress during grain filling of rice (Oryza sativa L.) are involved in occurrence of grain chalkiness. J. Plant Physiol.216, 52–57. 10.1016/j.jplph.2017.05.015 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Zhang, G. et al. Both short-term and long-term ozone pollution alters the chemical composition of rice grain. Bull. Env. Contam. Toxicol.113, 15. 10.1007/s00128-024-03927-5 (2024). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.


