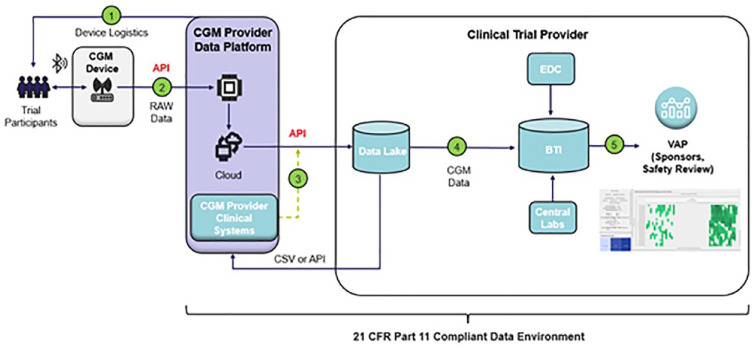
Figure 5.
CGM clinical trial data architecture and data flow. (1) study specific identification codes are provided for the CGM devices, (2) raw CGM data are transferred with the applicable identification codes, (3) blinded identification codes and CGM data are transferred to the Digital Health Platform via an API connection, (3) CGM data are joined with clinical trial based identifiers and loaded into the data lake, (4) CGM data are transferred into biostatistical systems for study based analysis, (5) data converted to CDISC SDTM format and incorporated with other subject data. Transferred into analysis, dashboards, tables, listings, and figures. Abbreviations: (API) application programming interface, (BTI) biostatistics technology infrastructure, (CSV) comma separated values files, (EDC) electronic data capture system, (VAP) visual analytics platform.